Discussion
Metastatic deposits to the small bowel are rare. Abbas (2007) and Minardi (1998) report that the majority of these lesions arise as a result of transcoelomic seeding of an intra-abdominal primary, of which colorectal cancer accounts for almost half of them. Other cancers include those of pancreatic, gastric and ovarian origins, with rarer occurrences from the lung, kidney and breast. Small bowel metastases from lung primaries are extremely uncommon, with an estimated reported incidence of 0.2-0.5% as reported in various case series by Berger (1999), Goh (2007) and Mcneill (1987). The absence of concomitant carcinomatosisperitonei in both of our cases may suggest the possible role of haematogenous seeding of tumour emboli.
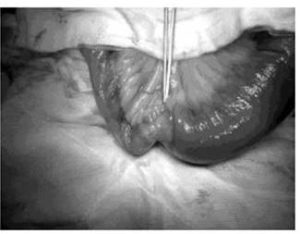
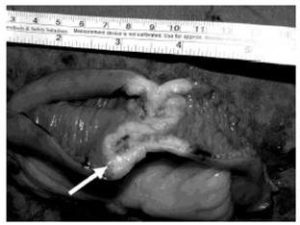
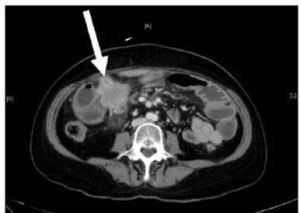
Small bowel tumours are notoriously difficult to diagnose due to their non-specific symptoms. They typically present as a surgical abdomen, with obstruction, perforation, or bleeding. These lesions usually pose significant diagnostic challenges. Facey et al (2007) reports that CT evaluation in detecting small bowel lesions has a sensitivity of 70%. It is often difficult to discern mural thickening in the presence of concomitant small bowel wall oedema, as was the case for our first patient. Some researchersrecommend capsule videoendoscopy for the assessment of the small bowel and subsequent double balloon endoscopy for biopsy of any visualized lesions. This is obviously not possible in an acute presentation. Mosier (1992) and Facey (2007) report that PET-CT is being used more frequently in the staging of certain tumours, with the benefit of identifying early metastatic disease, as seen in the second patient.
The role of surgical intervention in the management of patients with metastases to the small bowel remains palliative in nature. While some authors such as Han (2010) and Kant (2010) advocate comfort care in patients with widespread disease, surgical intervention remains integral in patients presenting with acute surgical emergencies as seen in our patients, both of whom had a reasonable life-expectancy from localized metastatic disease. The surgical options include a palliative bypass procedure or excisional resection. Complete macroscopic clearance has been recommended in selected patients with good functional status and isolated intra-abdominal disease as this improves the quality of life and symptom-free survival. Kant (2010) reports thatlong-term survival has been reported if complete clearance of intra-abdominal disease is attained.
The overall prognosis of metastatic lung tumours to the small bowel remains abysmal, with 5-year survival rates estimated at 9-20%. In comparison, the prognosis of metastatic tumours of the skin adnexa is unknown due to its rarity, but a 5-year-survival of 42% has been reported by Blake (2010).
Conclusions
Metastases from lung and skin adnexal malignancy to small bowel causing acute complications are rare. Surgical intervention in these patients remains integral,although the long term outcome remains dismal.
References
Abbas, S. M. & Merrie, A. E. H. (2007). “Resection of Peritoneal Metastases Causing Malignant Small Bowel Obstruction,” World J SurgOnco. 5:122.
Publisher – Google Scholar
Berger, A., Cellier, C., Daniel, C., Kron, C., Riquet, M., Barbier, J.- P., Cugnenc, P.- H. & Landi, B. (1999). “Small Bowel Metastases from Primary Carcinoma of the Lung: Clinical Findings and Outcome,” Am J Gastroenterol, 94(7) 1884-1887.
Publisher – Google Scholar – British Library Direct
Blake, P. W., Bradford, P. T., Devesa, S. S. & Toro, J. R. (2010). “Cutaneous Appendageal Carcinoma Incidence and Survival Patterns in the United States,” Arch Dermatol. 146 (6) 625-632.
Publisher – Google Scholar
Bulter, J. A., Cameron, B. L., Morrow, M., Kahng, K. & Tom, J. (1991). “Small Bowel Obstruction in Patients with a Prior History of Cancer,” Am J Surg, 162(6) 624-628.
Publisher – Google Scholar
Facey, K., Bradbury, I., Laking, G. & Payne, E. (2007). “Overview of the Clinical Effectiveness of Positron Emission Tomography in Selected Cancers,” Health Technol Assess. 11 (44) ii-iv, xi-267.
Publisher
Goh, B. K., Yeo, A. W., Koong, H. N., Ooi, L. L. & Wong, W. K. (2007). “Laparotomy for Acute Complications of Gastrointestinal Metastases from Lung Cancer: Is it a Worthwhile or Futile Effort?,” Surg Today 37 (5) 370-374.
Publisher – Google Scholar – British Library Direct
Han, S. L., Cheng, J., Zhou, H. Z. et al. (2010). “Surgically Treated Primary Malignant Tumour of Small Bowel: A Clinical Analysis,” World J Gastroenterol. 16 (12) 1527-1532.
Publisher – Google Scholar
Kant, K. M., Noordhoek, H. V. & Aerts, J. G. J. V. (2010). “A Patient with Four-Year Survival after Nonsmall Cell Lung Carcinoma with a Solitary Metachronous Small Bowel Metastasis,” J Oncol. 2010: 616130.
Publisher – Google Scholar
McNeill, P. M., Wagman, L. D. & Neifeld, J. P. (1987). “Small Bowel Metastases from Primary Carcinoma of the Lung,”Cancer, 59 (8) 1486-1489.
Publisher – Google Scholar
McNeil, P., Wagman, L. & Neifeld, J. (1987). “Small Bowel Metastases from Primary Carcinoma of the Lung,” Cancer, 59: 1486-1489.
Publisher – Google Scholar
Minardi, A. J., Zibari, G. B., Aultman, D. F., McMillan, R. W. & McDonald, J. C. (1998). “Small Bowel Tumours,” J Am CollSurg, 186:664-668.
Publisher – Google Scholar – British Library Direct
Mitsui, K., Tanaka, S., Yamamoto, H. et al. (2009). “Role of Double-Balloon Endoscopy in the Diagnosis of Small Bowel Tumours: The First Japanese Multicenter Study,” GastrointestEndosc. 70 (3) 498-504.
Publisher – Google Scholar
Molina, J. R., Yang, P., Cassivi, S. D., Schild, S. E. & Adjei, A. A. (2008). “Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment and Survivorship,” Mayo Clin Proc. 83 (5) 584-594.
Publisher – Google Scholar – British Library Direct
Mosier, D. M., Bloch, R. S., Cunningham, P. L. & Dorman, S. A. (1992). “Small Bowel Metastases from Primary Lung Carcinoma: A Rarity Waiting to be Found?,” Am Surg. 58 (11) 677-682.
Publisher – Google Scholar – British Library Direct
Trifan, A., Singeap, A. M., Cojocariu, C., Sfarti, C., Tarcoveanu, E. & Georgescu, S. (2010). “Single-Balloon Enteroscopy Following Videocapsule Endoscopy for Diagnosis of Small Bowel Tumours: Preliminary Experiences,” Chirurgia (Bucur) 105 (2) 211-217.
Publisher