Introduction
Bisphosphonates are synthetic analogue of inorganic pyrophosphates, which exert an inhibitory effect on osteoclast activity. They are used to treat diseases characterized by high bone resorption such as: multiple myeloma, osteolytic bone metastases, Paget’s disease of bone, fibrous dysplasia, McCune-Albright syndrome, hypercalcemia of tumour origin, treatment of osteoporosis.
Definition
One of the serious adverse effects of bisphosphonate therapy is bisphosphonate-associated osteonecrosis of the jaw. This is necrosis of the jaw bones persisting for more than 6 to 8 weeks, refractory to conservative treatment, observed in patients having no history of prior radiotherapy in the affected area, but treated with bisphosphonates – intravenously for at least one year, or orally for a much longer period, in relation to a general disease characterized by bone resorption.(American Association of Oral and Maxillofacial Surgeons 2007, Pechalova P. et al. 2011)
Theories of the Occurrence
The theories of the occurrence of bisphosphonate-associated osteonecrosis of the jaw are many, but none of them fully explains the mechanism of the disease. Hansen et al. (2006) believe that the development of infection associated with Actinomyces spp. and increased osteoclast numbers leads to osteonecrosis of soft tissue and bone. Reid еt al. (2007)reported that the toxicity of bisphosphonates on gingival mucosal cells is the cause for occurrence of osteonecrosis of the jaw. According to Allen and Burr (2009), the cause for occurrence of the disease is bone suppression as a result of accumulation of bisphosphonates. Roelofs et al. (2009) developed the theory of immunomodulatory effect of bisphosphonates on immunocompetent cells. Otto et al. (2010) discussed the possibility of local infection and changes in pH associated with it. Yamaguchi et al. (2010) postulated that inflammation caused by infection and/or tooth extraction could result in marked accumulation of inflammatory cells (granulocytes and/or macrophages), with these cells then causing destruction of part of jawbone. Oizumi T (2010) et al. found out that etidronate reduced the anti-bone-resorptive effects of alendronate. Etidronate also may inhibit the entry of nitrogen-containing bisphosphonates (NBPs) into cells related to inflammation and/or necrosis, and inhibit the binding of NBPs to bone hydroxyapatite, at least partly eliminate or substitute for NBPs that have already accumulated within bones, and thus if used as a substitution drug for NBPs, be effective at treating or preventing NBP-associated osteonecrosis of the jaw.
Localization
In most cases, bisphosphonate-associated osteonecrosis occurs in the mandible (Mast et al. 2012). There are several studies on the occurrence of the disease in the maxilla but no subgroup for examining the symptoms was defined(Carlson et al.2009, Estilo et al. 2008, Stockman et al. 2010).
Triggering Factors
It is assumed that the most common triggering factor for bisphosphonate-associated osteonecrosis of the jaw is extraction of teeth, periodontal diseases and traumas caused by dentures.
The risk is also related to bisphosphonate administration. By literature data, the potential of development ofosteonecrosis as a result of administration of aminobisphosphonates (Zoledronate and Pamidronate) is greater than that of non-aminobisphosphonates (Clodronate) (Bamias A. et al. 2005, Dannemann C. et al., 2007). Perhaps the most important factor for the occurrence of bisphosphonate-associated osteonecrosis of the jaw is the duration of treatment(Corso A.et al. 2007, Ruggiero SL et al.-2006, Ferlito S et al. 2012). Badros et al. (2006) suggest that with each year after diagnosing multiple myeloma and starting its treatment, the risk of developing bisphosphonate-associated necrosis of the jaw is increasing by 57%. Bamias A et al. (2005) suggest there is a strong correlation between the duration of treatment with bisphosphonates and manifestation of jaw osteonecrosis. They found that the risk of developing the condition varies from 1% 12 months after the start of treatment to 11% in the fourth year.
The authors reported that these figures vary depending on the type of bisphosphonate administered – in patients treated with Zoledronic acid only — from 1% in the first year to 21% in the third year since the beginning of the treatment, while in patients treated with Pamidronate with or without Zoledronic acid — from 0% in the first two years of treatment up to 7%after four years of treatment.
Stages of Disease
According to the American Association of Oral and Maxillofacial Surgery, development of bisphosphonate-associatedosteonecrosis of the jaw occurs in four stages:
Stage zero (0) – vague symptoms that may obscure the clinical picture, leading to delayed diagnosis.
In stage 1, bone exposure without any pain syndrome and signs of inflammation is observed.
In stage 2, bone exposure with pain syndrome and signs of inflammation is observed.
In stage 3, manifestation is characterized by bone exposure, pain and inflammation, and complications such as pathologic fracture, extraoral fistula, extension of the lesion to the base of the mandible and the floor of the maxillary sinus (AAOMS 2007, Ruggiero et al., 2009).
To the third stage, we add also sequester formation in the impaired area.
Recent studies assume that stabbing pain along the alveolar nerve is an early symptom significant for development of bisphosphonate-associated osteonecrosis of the mandible (Otto et al. 2009) , and occurrence of pain spreading to the maxillary sinus — for the maxilla (Mawardi et al. 2009), but we believe that in case of manifestation of such symptoms, a good understanding of the status of the patient is necessary, no previous radiotherapy in the maxillofacial area, and data on bisphosphonate administration for more than 6 months.
The study of Ferlito S et al. (2012) on 94 patients showed that formation of sequesters occurs an average of 8 months (5months for males and 9 months for females) after initiation of treatment with bisphosphonates.
Treatment
Treatment of bisphosphonate-associated osteonecrosis of the jaw is combined: conservative and surgical. Principles of conservative treatment have been clarified and it involves administration of antibiotics, antifungal and antiviral drugs to fight infection. Attention is also paid to the local oral hygiene maintained primarily with preparations containing chlorhexidine. Regarding surgical treatment, there is still controversy about the type of intervention and the time for performing the intervention. One group of authors (Ruggiero et al., 2004) recommends superficial curettage and prolonged conservative treatment. Another group (Agrillo et al., 2006) recommend hyperbaric oxygenation, surface debridement,laser, and in advanced cases – aggressive resection of the diseased bone and soft tissues. Hoff et al. (2008) reported healing process in 23% of patients treated with conservative therapy only.
Lazarovici et al. reported healing process after surface curettage in 82% of cases. Some authors perform complete removal of the diseased bone, cover it with adjacent mucosa and report good results in 85% – 100% of patients (Carlson E et al., 2009, Stockman P et al., 2010). Similar approach is used by another author (Otto S., 2012), who defined the boundaries of the diseased bone using fluorescent light. Ferlito S et al. (2012) observed 94 patients, in three of whom debridement was performed, and in 91 – sequestrectomy.
The analysis of numerous publications shows that in the early stages (AAOMS 1 and 2) more authors administers medication therapy and superficial curettage of the diseased bone, and in stage 3 medication therapy and sequestrectomy are administered. The method suggested by Otto S., et al (2012), which uses fluorescent light for visualization of vital bone, non-vital bone and microorganisms in the diseased bone is of interest. The authors argue that using this method it is possible to define the boundaries of non-vital bone and to remove it by resection in objectively evidenced boundaries.
Case Report
Female patient, aged 39, was referred for consultation with maxillofacial surgeon for lesions of the mucosa of the upper jaw with exposed bone structure in the area of left maxillary molars. The patient had complaints of pain in the left half of the maxilla and the maxillary sinus. Three years ago, treatment with bisphosphonate (Zoledronate) was initiated for bone metastases in the pelvis after surgery of breast carcinoma. Two years after the first administration of Zoledronate, tooth 28 was extracted because of severe pain. Extraction wound did not heal, pain in teeth 26 and 27 appeared, and those teeth were also extracted. Antibiotic treatment was administered with no success. This process lasted for 1 year. Objective status revealed a gingival lesion with dimensions 2/2.5 cm, with oedematous edges. The exposed bone in the area of teeth 26, 27 and 28 was yellowish and covered with purulent matter (Fig. 1). The bisphosphonate treatment was continued.
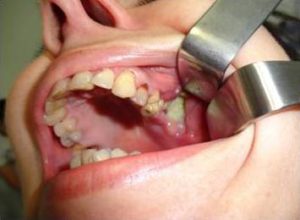
Fig. 1. Bisphosphonate–Associated Osteonecrosis of the Maxilla in the Area of Teeth 26, 27, 28
Radiographic studies (orthopantomography and CT) showed bone lesion in the area of left maxillary molars and complete opacification of the left maxillary sinus (Fig. 2, 3). The evidence of pain and unhealing lesions of the maxilla, the necrosis of the alveolar bone of the maxilla and the data on the use of bisphosphonates for metastatic breast cancers gave us grounds to diagnose bisphosphonate-associated necrosis of the maxilla — AAOMS stage 3.
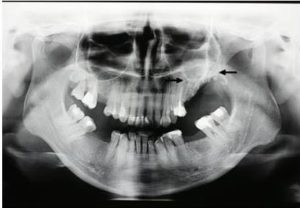
Fig. 2. Preoperative Ortopanthomogram
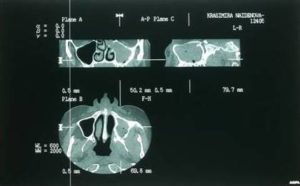
Fig. 3. Preoperative CT Scan
The following treatment was administered:
Medications in empiric order were prescribed 3 days prior to surgery: Unasyn, Metronidazole, Diflucan and Eludril for oral care. Sequestrectomy was performed under general anesthesia (Fig. 4).
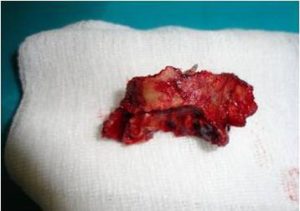
Fig. 4. Bone Sequester Consisting of the Alveolar Ridge and the Floor of the Maxillary Sinus
The surgical margins were defined macroscopically without usage of fluorescent light. The removal of the sequester revealed maxillary sinus filled with hypertrophied inflamed mucosa (Fig. 5). Radical removal of the altered mucosa was performed. The oroantral communication formed was closed with mucosal flap taken from the buccal mucosa (Fig. 6). Antimicrobial therapy was administered for 5 days after surgery. Sutures were removed on day 10. Surgical wound healed primarily.
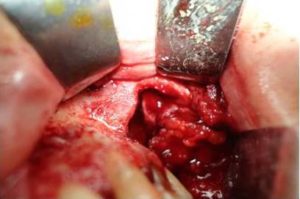
Fig. 5. Maxillary Sinus is Filled with Hypertrophied Mucosa
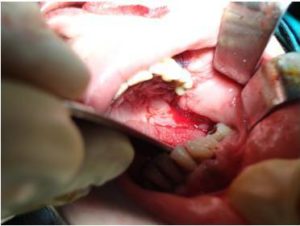
Fig. 6. Plastic Closure of Oroantral Defect with Buccal Mucosa
Histological examination of the bone sequester (after two-day decalcification) showed the existence of actinomycetedruses (fig. 7), sinus mucosa with polyp growth, hypertrophy, severe inflammation (Fig. 8), gingival mucosa withpronounced inflammation, hyperplastic changes, in some places to the extent of pseudoepitheliomatosis andhyperkeratosis (Fig. 9).
Follow-up examination one month later found healed surgical wound without any subjective complaints.
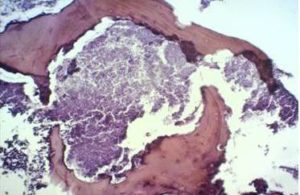
Fig. 7. Histological Examination of the Bone Sequester – Actinomycotic Osteomyelitis
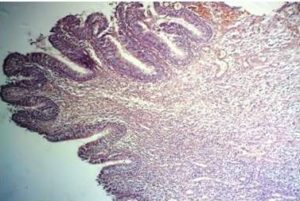
Fig. 8. Histological Examination of the Sinus Mucosa – Polyp Growth, Hypertrophy and Severe Inflammation
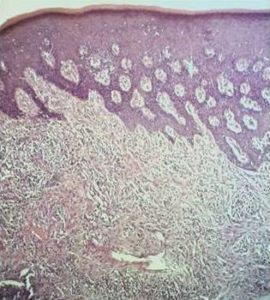
Fig. 9. Histological Examination of the Gingival Mucosa — Pronounced Inflammatory Process with Hyperplastic Changes
Discussion
Bisphosphonate-associated osteonecrosis of the jaw is a serious complication after bisphosphonate therapy for diseases characterized by high bone resorption. Treatment of this complication is a major challenge for oncologists and oral and maxillofacial surgeons. Principles of conservative treatment have been clarified – it involves administration of antibiotics,antifungal and antiviral drugs to fight infection. In our opinion inflammation in soft tissues precedes osteonecrosis. That’s why administration of antimicrobial therapy was appropriate in every case of bisphosphonate-associated osteonecrosis of the jaws. Surgical treatment is still controversial among surgeons in the part concerning the volume of surgery and the time of its performance. The literature review shows that in AAOMS stages 1 and 2, most of the authors administer medication therapy and superficial curettage of the diseased bone, while extended resection of the diseased bone until reaching healthy tissue is indicated in stage 3, and in case of sequester – sequestrectomy is obligatory. The case described shows that the involvement of the maxilla and the maxillary sinus requires sequestrectomy or resection of the necrotic bone, radical maxillary sinus surgery and plastic closure of the formed oroantral communication. We believe that surgical treatment was necessary in described case because there was no other way to create condition for healing process in surrounded soft tissues.
The result of histological examination of the bone sequester indicates that actinomycetes play an important role in the development of bisphosphonate-associated necrosis of the jaw, and the changes in sinus mucosa and gingiva correspond to severe inflammation process. The induced necrosis of the bone is likely to create favorable conditions for worsened trophism of the periosteum and adjacent soft tissues, thus being a prerequisite for the occurrence of soft tissue lesion.
The described case of successful surgical treatment of bisphosphonate-associated osteonecrosis of the maxilla(AAOMS stage 3) with maxillary sinusitis illustrates the widening range of prospects for treatment of the condition.Further observations to specify the indications and parameters for surgical treatment in a larger population of patients with bisphosphonate-associated osteonecrosis of the jaw are necessary.
References
Agrillo, A., Petrucci, M. T., Tedaldi, M., et al. (2006). “New Therapeutic Protocol in the Treatment of Avascular Necrosis of the Jaws,” J Craniofac Surg 17:1080-1083.
Publisher – Google Scholar
Allen, M. R. & Burr, D. B. (2009). “The Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaw: So Many Hypotesis, So Few Data,” J Oral Maxillofac Surg 67: 61-70.
Publisher – Google Scholar
American Association of Oral and Maxillofacial Surgeons Position Paper on Bisphosphonate-Related Osteonecrosis of the Jaws (2007). J Oral Maxillofac Surg 65: 369-376.
Publisher
Badros, A., Weikkel, D., Salama, A., et al. (2006). “Osteonecrosis of the Jaw in Multiple Myeloma Patients: Clinical Features and Risk Factors,” J Clin Oncol 24: 945 — 952.
Publisher – Google Scholar
Bamias, A., Kastritis, E., Bamia, C., et al. (2005). “Osteonecrosis of the Jaw in Cancer after Treatment with Bisphosphonates: Incidence and Risk Factors,” J Clin Oncol 23: 8580 — 8587.
Publisher – Google Scholar
Carlson, E. R. & Basile, J. D. (2009). “The Role of Surgical Resection in the Management of Biphosphonate Related Osteonecrosis of the Jaws,” J Oral Maxillofac Surg 67: 85-95.
Publisher – Google Scholar
Corso, A., Varettoni, M., Zappasodi, P., et al. (2007). “A Different Schedule of Zoledronic Acid Can Reduce the Risk of the Osteonecrosis of the Jaw in Patients with Multiple Myeloma,” Leukemia: 1 — 4.
Publisher – Google Scholar
Dannemann, C., Gratz, K. W., Riener, M. O., et al. (2007). “Jaw Osteonecrosis Related to Bisphosphonate Therapy,” ASevere Secondary Disorder. Bone 40: 828 — 834.
Publisher – Google Scholar
Estilo, C. L., Van Poznak, C. H., Wiliams, T., et al. (2008). “Osteonecrosis of the Maxilla and Mandible in Patients with Advanced Cancer Treated with Bisphosphonate Therapy,” Oncologist 13: 911-920.
Publisher – Google Scholar
Ferlito, S., Puzzo, S., Palermo, F., et al. (2012). “Treatment of Bisphosphonate-Related Osteonecrosis of the Jaws: Presentation of a Protocol and an Observational Longitudinal Study of an Italian Series of Cases,” British Journal of Oral and Maxillofacial Surgery 50: 425-429.
Publisher – Google Scholar
Hansen, T., Kunkel, M., Weber, A., et al. (2006). “Osteonecrosis of the Jaw in Patient Treated with Bisphosphonates-Histomorphologic Analysys in Comparison with Infected Osteoradionecrosis,” J Oral Pathol Med 35: 155-160.
Publisher – Google Scholar
Hoff, A. O., Toth, B. B., Altundag, K., et al. (2008). “Frequency and Risk Factor Associated with Osteonecrosis of the Jaw in Cancer Patients Treated with Intravenous Bisphosphanates,” J Bone Miner Res 23: 826-836.
Publisher – Google Scholar
Lazarovici, T. S., Yahalom, R., Taicher, S., et al. (2009). “Bisphosphonate Related Osteonecrosis of the Jaws: A Single-Center Study of 101 Patients,” J Oral Maxillofac Surg 67: 850-855.
Publisher – Google Scholar
Mast, G., Otto, S., Mücke, T., et al. (2012). “Incidence of Maxillary Sinusitis and Oro-Antral Fistulae in Bisphosphonate-Related Osteonecrosis of the Jaw,” J Cranio-Maxillo-Facial Surg 40: 568-571.
Publisher – Google Scholar
Mawardi, H., Treister, N., Richardson, P., et al. (2009). “Sinus Tract-an Early Sign of Bisphosphonate-Associated Osteonecrosis of the Jaws?,” J Oral Maxillofac Surg 67: 593-601.
Publisher – Google Scholar
Oizumi, T., Funayama, H., Yamaguchi, K., et al. (2010). “Inhibition of Necrotic Actions of Nitrogen-Containing Bisphosphonates (Nbps) and Their Elimination from Bone by Etidronate (A Non-NBP): A Proposal for Possible Utilization of Etidronate as a Substitution Drug for Nbps,” J Oral Maxillofac Surg 68: 1043-54.
Publisher – Google Scholar
Otto, S., Hafner, S., Mast, G., et al. (2010). “Bisphosphonate-Related Osteonecrosis of the Jaw: Is PH the Missing Part in the Pathogenesis Puzzle?,” J Oral Maxillofac Surg 68: 1158-1161.
Publisher – Google Scholar
Otto, S., Schreyer, C., Hafner, S., et al. (2012). “Bisphosphonate-Related Osteonecrosis of the Jaws-Characteristics, Risk Factor, Clinical Features, Localization and Impact on Oncological Treatment,” Journal of Cranio-Maxillo-Facial Surgery 40: 303-309.
Publisher – Google Scholar
Pechalova, P., Bakardjiev, A., Zaprianov, Z., et al. (2011). “Bisphosphonate-Associated Osteonecrosis of the Jaws-Report of Three Cases in Bulgaria and Review of the Literature,” Acta Clinica Croatica 50: 273-279.
Publisher – Google Scholar
Reid, I. R., Bolland, M. J. & Grey, A. B. (2007). “Is Bisphosphonate Associated Osteonecrosis of the Jaw Caused by Soft Tissue Toxicity?,” Bone 41: 318-320.
Publisher – Google Scholar
Roelofs, A. J., Jauhiainen, M., Mönkkönen, H., et al. (2009). “Peripherial Blood Monocytes are Responsible for Gammaselta T Cell Activation Induced by Zolendronic Acid through Accumilation of IPP/DMAPP,” Br. J. Haematol 144: 245-250.
Publisher – Google Scholar
Ruggiero, S. L., Fantasia, J. & Carlson, E. (2006). “Bisphosphonate-Related Osteonecrosis of the Jaw: Background and Guidelines for Diagnosis, Staging and Management,” Oral Surg, Oral Med, Oral Pathol Oral Radiol Endodod 102: 433-441.
Publisher – Google Scholar
Stockmann, P., Vairaktaris, E., Wehrhan, F., et al. (2010). “Osteotomy and Primary Wound Closure in Bisphosphonate-Associated Osteonecrosis of the Jaw: A Prospective Clinical Study with 12 Month Follow-Up,” Support Care Cancer 18: 449-460.
Publisher – Google Scholar
Yamaguchi, K., Oizumi, T., Funayama, H., et al. (2010). “Osteonecrosis of the Jawbones in 2 Osteoporosis Patients Treated with Nitrogen-Containing Bisphosphonates: Osteonecrosis Reduction Replacing NBP with Non-NBP (Etidronate) and Rationale,” J Oral Maxillofac Surg. 68: 889-97.
Publisher – Google Scholar











