Abstract
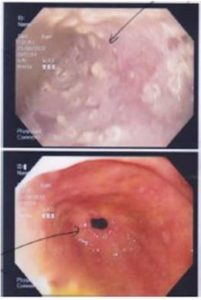
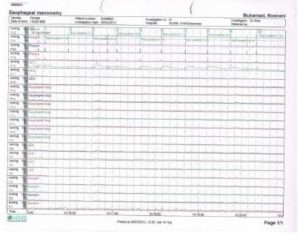
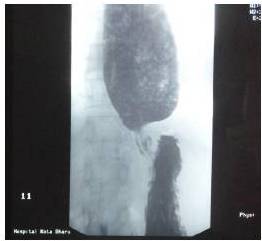
Achalasia cardia is a benign idiopathic disorder caused by progressive neuronal degeneration in the mesenteric plexus of Auerbach, which causing non-relaxing, hypertensive lower esophageal sphincter (LES) and aperistalsis of the esophageal body. We report a 52-year-old lady presented with progressive dysphagia for 4 months, associated with loss of weight. Other than underweight with BMI of 18kg/m2 and mild tenderness at epigastrium, clinical examinations were unremarkable. Upper endoscopy revealed dilated esophagus with evidence of fungal esophagitis, and severe gastritis. Esophageal manometry confirmed achalasia cardia with evidence of aperistalsis of esophageal body and failure of relaxation of lower esophageal sphincter (LES) on swallowing. She had undergone pneumatic dilatation for 3 times before significant improvement in symptoms noticed clinically. Literatures were reviewed to compare current available therapies for achalasia cardia and it is recommended that the patient should undergo laparoscopic myotomy and partial fundoplication (to prevent free reflux of gastric acid into the esophagus) for better remission. Other treatment modalities such as Botulinum toxin injection and pneumatic dilation can offer dysphagia control, but they are temporary and reversible measures. The objective of this case report is to review the current available treatment modalities for the management of achalasia cardia.