Discussion
Due to the marked clinico-radiological and histological similarity between tuberculosis and sarcoïdosis, and the high prevalence of tuberculosis in our country, confusion may occur and the patients may receive unuseful repeated courses of anti-tubercular therapy [Farheen et al., 2011].
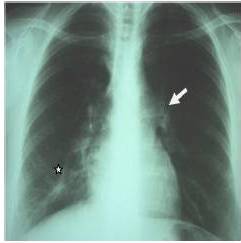
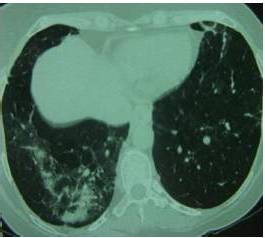
Sarcoïdosis is a multisystem disorder of unknown cause, characterized by non specific constitutional symptoms such as fever, weakness and weight loss. Commonly, the disease involves mediastinal lymph nodes and lungs (90% of patients) but any other systems may be involved [Goksel et al., 2011].The chest X-ray is abnormal in 85 to 95% of cases. It commonly shows hilar and mediastinal lymphadenopathy. Other characteristic findings include interstitial lung disease, retractions and irregular opacities. Computed-tomography scan of the thorax shows thickening of bronchovascular bundles and perilymphatic distribution of nodules which represent the sarcoïd granuloma [Valeyre et al., 2005]. At the stage of fibrosis: bronchial distortion, hilar retraction, diaphragmatic ascension and bronchectasies are noted. Perihilar masses rarely evolve toward the excavation. Aspects of ray honey, ground glass opacities and bubbles are less frequently observed [Valeyre et al., 2005]. The tuberculin anergy is present in 75 % of cases. Generally there is no biological inflammatory syndrome. The hypergammaglobulinaemia is common. Lymphopenia, hypercalciuria, more rarely hypercalcemia may contribute to the diagnosis. The diagnostic value of ECA level is low [Pavic et al., 2008]. At BAL, lymphocytes rate over than 30 % (normal = 10 — 15 %) increases the probability of sarcoïdosis.
Histopathologically, sarcoïdosis is characterized by the presence of non-caseating granuloma. Rarely, non-caseating necrosis may be noted on sarcoïdosis granuloma, in this case little and fibrinoid [Pavic et al., 2008].
At initial presentation the epidemiological and clinical features, presence of necrosis in side granuloma could very well be matched with the diagnosis of tuberculosis. Presence of cavity formation, common in TBC and uncommon in sarcoïdosis, strengthens this hypothesis. Repeated courses of ATT with whole duration of 25 months were instituted while lung damage continues to progress with new radiological findings more suggestive of sarcoïdosis such as peripheral nodules and distortion of pulmonary architecture with bronchectasies.
After oriented check-up, it has been clear that the patient had many features of sarcoïdosis, with usually negative reaction to tuberculin and no evidence of active tuberculosis. Mycobacterium tuberculosis was never isolated. Finally the diagnosis was redressed and the favorable evolution with corticosteroid therapy confirms this hypothesis.
Recently, adding to the diagnostic difficulties, there are also some reports of coexistence of sarcoïdosis and tuberculosis in the same patients [Abdurrahman et al., 2006]. Tuberculosis has been described as both preceding and coexisting with sarcoïdosis, and as an opportunistic infection in patients with sarcoïdosis who are on corticosteroids, which depress the cell-mediated immunity. Only a few cases of TBC in patients with sarcoïdosis have been reported in the world literature, and all of them had a course of corticosteroids or were immunosuppressed. Supriya and al (2010) relate a case of a patient, considered the unique in the literature, with sarcoïdosis, who had never taken corticosteroids and who develops tuberculosis. Fite and al (2006) demonstrated that M. tuberculosis DNA was present in 9/23 sarcoïdosis biopsies (39 %), in 1/23 control patients (4%) (p = 0.01), and in all tissue samples from 12 control patients with tuberculosis. These results suggest a causality link between M. tuberculosis infection and sarcoïdosis in some cases.
It is possible that a coincidental reactivation of tuberculosis with acute sarcoïdosis occurred. However, in some cases there may be an etiological relationship between sarcoïdosis and mycobacterial infection, and it is possible that clinical manifestations of sarcoïdosis may have been a response to infection with M. tuberculosis [Uddenfeldt et al., 1982].
Conclusion
Clinical and radiological features of tuberculosis and sarcoïdosis are quite similar. If we have no bacteriological nor histological proof for tuberculosis or in case of no improvement after ATT, diagnosis may be quickly reconsidered.
References
Abdurrahman, T., Ortenca, R., Arzu, S., Arzu, T., Ibrahım, H. & Lutfi, Ç. (2006). “Concurrent Presentations of the Sarcoidosis, Tuberculosis and Lymphoma in a Single Patient,” Respiratory Medicine, 100, 951—953.
Publisher – Google Scholar
Badar, F., Azfar, S. F.,Ahmad, I.,Yasmeen, S. & Kirmani, S. (2011). “Diagnostic Difficulties in Differentiating Sarcoidosis from Tuberculosis,” Oman Medical Journa, 26 (3), 210-211.
Publisher – Google Scholar
Fité, E., Fernández-Figueras, M. T., Prats, R., Vaquero, M. & Morerea, J. (2006). “High Prevalence of Mycobacterium Tuberculosis DNA in Biopsies from Sarcoidosis Patients from Catalonia, Spain,” Respiration, 73, 20—26.
Publisher – Google Scholar
Kiter, G.,Müsellim, B.,Cetinkaya, E.,Türker, H., et al. (2011). “Clinical Presentations and Diagnostic Work-up in Sarcoidosis: A Series of Turkish Cases (Clinics and Diagnosis of Sarcoidosis,” Tüberküloz ve Toraks Dergisi, 59 (3), 248-258.
Publisher – Google Scholar
Pavic, M., Le Pape, E., Debourdeau, P., Rabar, D., Crevon, L. & Colle, B. (2008). “Granulomatoses Systémiques Pseudosarcoïdosiques D’étiologie Déterminée et non Tuberculeuse. Etude de 67 Observations,” La Revue de médecine interne, 29, 5—14.
Publisher – Google Scholar
Sarkar, S., Saha, K. & Das, C. S. (2010). “Isolated Tuberculous Liver Abscess in a Patient With Asymptomatic Stage I Sarcoidosis,” Respiratory Care, 55 (12), 1751—1753.
Publisher – Google Scholar
Uddenfeldt, M., Stjemberg, N. & Lundgren, R. (1982). “Sarcoidosis or Tuberculosis — A Case Report,” Tubercle, 63, 221-223.
Publisher – Google Scholar
Valeyre, D., Nunes, H., Duperron, F., Soler, P., Kambouchner, M. & Brauner, M. (2005). “Sarcoïdose,” EMC-Pneumologie2, 147—164.
Publisher – Google Scholar