Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing, low-grade sarcoma of dermal fibroblastic origin. It was first described by Darier and Ferrand in 1924 and was referred to as a progressive and recurrent dermatofibroma. It was later officially termed ‘dermatofibrosarcoma protuberans’ by Hoffmann in 1925 as stated by Torreggiani W.C et al (2002) and Lim E.K et al (2008).
In research review by Torreggiani W.C et al (2002), dermatofibrosarcoma protuberans typically arises in the dermis as a multinodular mass, which then spreads into the subcutaneous tissues and muscle. The tumour occurs in patients of all ages, with the highest frequency occurring between the second and the fifth decades as mentioned by Mehrany K et al (2006) and Torreggiani W.C et al (2002). The trunk is the most common site involved accounting for almost half of all cases by Torreggiani W.C et al (2002) and Lim E.K et al (2008). In two studies by Gatlin J.L et al (2011) and Torreggiani W.C et al (2002), the extremities followed by the head and neck are the next most common sites, but tumours may occur on any part of the body. Males are more frequently affected as reported by Torreggiani W.C et al (2002) and Korkolis D.P et al (2007). Despite being slow growing and having low potential for metastasis, the treatment for dermatofibrosarcoma protuberans remains challenging. Wide local excision, Mohs surgery, and a multidisciplinary combination approach, selected based on both tumour size and patient factors, were capable of achieving very high local control rates in the treatment of DFSP as mentioned by DuBay D et al (2004).
Case 1
A 40 year-old Chinese lady presented with a left breast lump which is progressively increased in size over a period of 4 years. She did not seek treatment initially as the lump was painless and was forgotten about, until recently when she palpated it. Her family and medical history were unremarkable.
On examination, there was a 3.0 x 2.5 cm firm and non-tender mass at the upper outer quadrant of the left breast. The mass was superficial and mobile. The overlying skin was adherent with purplish discoloration. No regional lymph nodes were palpable. Bilateral mammogram of the breasts were done and showed superficial soft tissue density at the upper outer quadrant of the left breast (Figure 1).
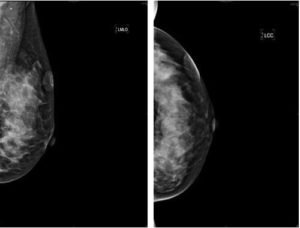
Figure 1: Benign Mammographic Findings of a Superficial Soft Tissue Density at Upper Outer Quadrant of the Left Breast
An ultrasound was also performed as the breast parenchyma pattern was dense on mammogram, revealing a well-defined oval hypoechoic lesion within the subcutaneous tissue (Figure 2). However, the radiological features were not suggestive of malignancy. Pre operative differential diagnosis was early breast carcinoma and benign breast lesion.
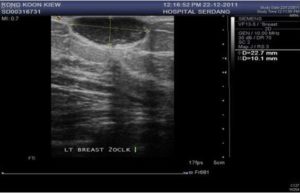
Figure 2: Ultrasound Findings of a Well Defined Oval Hypoechoic Lesion Measuring 2.2 X 1.0 Cm.
We performed an excision biopsy of the left breast lump since the clinical and radiological features were benign. The gross pathology of the specimen showed a greyish fibrous tissue measuring 3.5 x 2.3 x1.0 cm. Histological examination revealed an intradermal tumour composed of spindle cells arranged in storiform pattern with infiltration of the subcutaneous fatty tissue. There was only mild mitotic activity (3-5 per 10 high-power fields). On immunohistochemical studies, the tumour stained positive for CD34. Unfortunately the resection margin was involved. A diagnosis of dermatofibrosarcoma protuberans was eventually made.
As the resection margin was involved, she underwent a wide excision of the surgical scar with a gross margin of 2 cm and the defect was closed primarily. Histological examination showed a tiny focus of the spindle cell tumour arranged in storiform pattern. Mitosis was not seen and all margins were clear from tumour infiltration. These findings confirmed the complete excision of the residual tumour.
She was seen by the oncologist and is currently not undergoing adjuvant treatment in view of the clear surgical margin of resection. She is currently under close follow-up with yearly mammogram as surveillance.
Case 2
A 31 year-old Burmese lady presented with a 12 years history of a lump in the anterior abdominal wall which gradually enlarged with no associated symptoms. She had the lump excised at another hospital 3 years prior to her presentation to us. She provided us a copy of the histopathology report which was reported as low grade spindle cell sarcoma. She did not undergo any further treatment there after her surgery, and noticed a recurrence of the lump at the surgical site a year ago. On examination, there was a large and hard mass about 10 x 8 cm at the right hypochondrium. The mass was lobulated, not mobile and not tender.
Magnetic resonance imaging (MRI) of the abdomen revealed a lobulated mass measuring 8.2 x 4..3 x 7.4 cm of isointense signal on T1 weighted film (Figure 3), slightly hyperintense on T2 weighted film (Figure 4) and not suppressed in the fat suppression sequence.
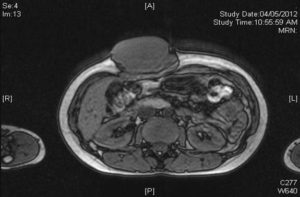
Figure 3: MRI of Abdomen Showing a Lobulated Mass at Anterior Abdominal Wall with Isointense Signal on T1 Weighted Film
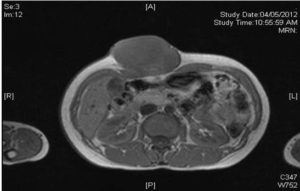
Figure 4: MRI of Abdomen Showing the Same Lobulated Mass at the Anterior Abdominal Wall with a Slight Hyperintense Signal on T2 Weighted Film.
Following Gandolinium administration, there is heterogeneous contrast enhancement predominantly at the periphery of the lesion with preservation of fat plane between the mass and rectus abdominis muscle (Figure 5). These features were suggestive of a soft tissue tumour with differential diagnosis were desmoids tumour or soft tissue sarcoma.
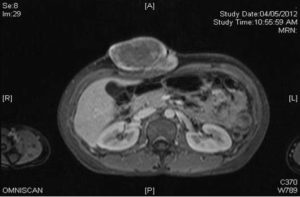
Figure 5: MRI of Abdomen Showing a Lobulated Mass at Anterior Abdominal Wall with Heterogenous Enhancement Predominantly Periphery after Gandolinium Contrast Administration
A wide excision of the lump was performed and the defect was closed primarily. Intraoperatively, the tumour was attached to the rectus abdominis muscle. The gross pathology of the specimen showed a circumscribed firm whitish tumour measuring 9.0 x 6.5 x 6.0 cm with no infiltration into the overlying skin. Histology examination revealed a tumour composed of spindle shaped cells arranged in storiform patterns and exhibiting moderate pleomorphism. Mitotic activity ranges from 2-5 per 10 high power fields. The tumour is seen < 1 mm away from the superior, inferior and deep resection margins. On immunohistochemistry studies, the tumour was positive for CD34 and Vimentin. She is currently well and still undergoing radiotherapy to the surgical site.
Discussion
Dermatofibrosarcoma protuberans (DFSP) represents a spectrum of mesenchymal spindle cell tumours that typically involve both dermis and subcutaneous. Clinically, DFSP presents as a firm, lobulated cutaneous mass that gradually increase in size over a prolonged period of time, often years. The slow rate of tumour growth is characteristic and there is often a delay in presentation as patients usually ignore the tumour due to its indolent nature. Occasionally, there is a history of preceding trauma as observed by Park T.H et al (2011).
In research by Korkolis D.P et al (2007), they revealed that the histological features of DFSP are characterized as a storiform or ‘cartwheel’ pattern of fusiform cells with no nuclear atypia or increased mitotic activity. Infiltration of the dermis and surrounding fat in combination with the clinical picture are highly suggestive of the diagnosis as observed Korkolis D.P et al (2007). The infiltrative feature into the dermis by strands of tumour extends far beyond the apparent clinical margins and gives rise to the characteristic high local recurrence rate after standard surgical excision.
Several histological subtypes have been described: pigmented (Bednar tumour), myxoid, myoid, sclerosing, with granular cells, with multinucleated giant cells (resembling giant cell fibroblastoma), atrophic and with fibrosarcomatous areas by Korkolis D.P et al (2007). Immunohistochemical staining, particularly CD34 and Vimentin, have been proven to be very important for the accurate diagnosis of DFSP noted again by Korkolis D.P et al (2007). CD34 has a sensitivity that ranges from 84% to 100% supported by Gatlin J.L et al (2011).
A study by Powers C.N et al (1993) found that fine needle aspirates of DFSP yield moderately cellular smears with discohesive cells as well as tiny fragments of tissue which may be large enough to process as a cell block. They demonstrate the storiform arrangement of cells in the stromal fragments. In addition to the fibrohistiocytic cells arranged in a storiform array, background elements are also important and help narrow the differential. Among these features are the presence of entrapped adipose tissue, a delicate network of capillaries, and the absence of inflammatory cells as mentioned by Powers C.N et al (1993).
The role of imaging in DFSP is to assess local extent of the tumour and to identify any distant metastasis. It may also assist in the surveillance for recurrence. On magnetic resonance imaging, T1 low signal and T2 high signal are consistent features with enhancement seen after gadolinium seen by Gatlin J.L et al (2011) and Torreggiani W.C et al (2002). Hence, CT scan has limited value except for assessment of bone involvement as stressed by Park T.H et al (2011).
The rate of recurrence after DFSP excision varies widely in the literature. A review of the literature published in 1996 found a mean recurrence rate of 18% (range, 0—60%) after treatment with wide local excision in 15 studies by DuBay D et al (2004). Szollosi Z et al (2005) mentioned that distant metastasis (1 % – 4%) and tumour related deaths are extremely rare. Re-excision of tumour positive areas until tumour-free margins are obtained or ‘histographical surgery’ ensures a high cure rate of up to 97% while preserving normal tissue as mentioned by Park T.H et al (2011). Park T.H et al (2011) also concluded that histological subtype, high mitotic index, cellularity, size, location on the head and neck, and recurrent lesions are factors reportedly associated with higher recurrence rates. Evidently, the most important factor for local control is obtaining negative surgical margins. Our two cases that underwent conventional wide margin resection shown comparable outcomes as the latter case of abdominal wall DFSP was given radiotherapy in view of moderate histological features. Both of cases were closely surveillance without any recurrence in two years time.
Mohs micrographic surgery (MMS) has emerged as an alternative approach allowing immediate microscopic examination of the margins. The average recurrence rate of dermatofibrosarcoma protuberans treated by Mohs micrographic surgery is less than 2% as reviewed by Mehrany K et al (2006). However, MMS requires considerable training, a specialized team, and multiple stages of surgery. Last study by Foroozan et al (2012) found out a mean raw recurrence rate of 1.03% after Mohs micrographic surgery among 19 nonrandomized non comparative trials.
Mehrany K et al (2006) argued that chemotherapy is not useful in the treatment of localized DFSP. It may have a role in metastatic disease, but significant benefits remain to be demonstrated. Recently, the tyrosine kinase inhibitor, Imatinib, was shown to induce regression of advanced DFSP when it is impossible to obtain surgical margins stressed by Mehrany K et al (2006). Although the results are promising, further trials are still required.
Adjuvant radiation therapy can be administered if margins are positive or very close to the tumour after maximal resection as mentioned by Park T.H et al (2011). DFSP spreads by a haematogenous route, with the lung being the most common site of metastasis. Regional lymph nodes are rarely involved. Therefore, prophylactic lymphadenectomy is not recommended as lymphatic metastases from DFSP are rare.
Conclusion
Dermatofibrosarcoma protuberans is an unusual cutaneous tumour from different ectoderms origin with a tendency to recur locally after inadequate management. Histopathological confirmation can be difficult and differential diagnosis should include specific immunohistochemical studies. Wide surgical resection with microscopically healthy margins and appropriate reconstruction remains the cornerstone of treatment.
References
DuBay, D., Cimmino, Y., Lowe, L., Johnson, T. M. & Sondak, V. K. (2004). “Low Recurrence Rate after Surgery for Dermatofibrosarcoma Protuberans: A Multidisciplinary Approach from a Single Institution,” American Cancer Society;100(5): 1008-1016.
Publisher – Google Scholar – British Library Direct
Foroozan, M., Sei, J. F., Amini, M., Beauchet, A. & Saiag, P. (2012). “Efficacy of Mohs Micrographic Surgery for the Treatment of Dermatofibrosarcoma Protuberans: Systematic Review,” Arch Dermatol; 148(9):1055-63.
Publisher – Google Scholar
Gatlin, J. L., Hosch, R. & Khan, M. (2011). “Dermatofibrosarcoma Protuberans of the Scalp with Fibrosarcomatous Degeneration and Pulmonary Metastasis,” J Clin Imaging Sci; 1:55.
Publisher – Google Scholar
Korkolis, D. P., Liapakis, I. E. & Vassilopoulos, P. P. (2007). “Dermatofibrosarcoma Protuberans: Clinicopathological Aspects of an Unusual Cutaneous Tumor,” Anticancer Research; 27: 1631-1634.
Publisher – Google Scholar – British Library Direct
Lim, E. K., Chia, H. L., Yu, T. J. & Chang, H. C. (2008). “Suprapubic Dermatofibrosarcoma Protuberans: Case Report and Literature Review,” JTUA; 19(4): 232-234.
Publisher – Google Scholar
Mehrany, K., Swanson, N. A., Heinrich, M. C., Weenig, R. H., Lee, K. K., White, C. R. Jr, & Devere, T. S. (2006). “Dermatofibrosarcoma Protuberans: A Partial Response to Imatinib Therapy,” Dermatol Surg; 32(3): 456-459.
Publisher – Google Scholar
Park, T. H., Seo, S. W., Kim, J. K. & Chang, C. H. (2011). “Reconstructive Challenge of Dermatofibrosarcoma Protuberans in the Female Breast,” World Journal of Surgical Oncology, 9(1).
Publisher – Google Scholar
Powers, C. N., Hurt, M. A. & Frable, W. J. (1993). “Fine-Needle Aspiration Biopsy: Dermatofibrosarcoma Protuberans,”Diagnostic Cytopathology; 9(2): 145-150.
Publisher – Google Scholar – British Library Direct
Szollosi, Z. & Nemes, Z. (2005). “Transformed Dermatofibrosarcoma Protuberans: A Clinicopathological Study of Eight Cases,” J Clin Pathol; 58: 751—756.
Publisher – Google Scholar
Torreggiani, W. C., Al-Ismail, K., Munk, P. L., Nicolaou, S., O’Connell, J. X. & Knowling, M. A. (2002). “Dermatofibrosarcoma Protuberans: MR Imaging Features,” AJR; 178: 989—993.
Publisher – Google Scholar – British Library Direct







