Introduction
Renal cell carcinoma with metastasis to ureter is extremely rare. We report a case of metachronous metastasis of renal cell carcinoma to ipsilateral ureteric stump after radical nephrectomy. A literature review was done to evaluate the disease nature, proposed mechanism of spread, and the management of other reported cases.
Case
An 81-year-old man presented with gross painless haematuria for one month. Ultrasound scan showed a renal mass at upper pole of right kidney. Urine cytology did not show any malignant cells. Flexible cystoscopy showed no bladder lesion. Contrast computed tomography (CT) scan revealed a 7.5 x 6.3 x 6.3 cm renal cell carcinoma (RCC) over upper pole of right kidney. (Figure 1) Invasion of right renal vein and renal calyces were present. The inferior vena cava was not involved. Open radical nephrectomy and thrombectomy were performed. Pathology was clear cell type RCC, Fuhrman grade 3. The tumour was confined to the kidney by the renal capsule with no perinephric fat or renal sinus fat infiltrate. Ureteric resection margin was clear (pT3aN0M0, AJCC 7th edition 2010).
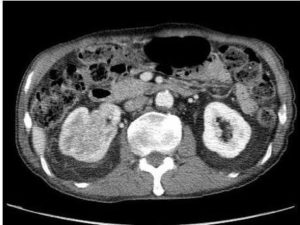
Figure 1: CT Scan Showing Rt Renal Tumour
He complained of gross painless haematuria again one year later. Flexible cystoscopy showed a 4cm tumour over right lateral bladder wall with narrow stalk protruding from the right ureteric opening. Transurethral resection was done and the pathology was clear cell type RCC, Fuhrman grade 4. Contrast CT scan was done one month after the transurethral resection. It showed the right ureteral stump was distended down to right vesicoureteric junction with suspicious soft tissue mass at right vesicoureteric junction (Figure 2). A 3cm segment of suspicious eccentric wall thickening was also incidentally found at distal ascending colon. Colonoscopy confirmed the colonic tumour over hepatic flexure. Biopsy result was adenocarcinoma. Chest X-ray was clear and bone markers were normal. Clinically, it was a metachronous metastasis of renal cell carcinoma at ipsilateral ureteric stump together with a synchronous adenocarcinoma of ascending colon. Open completion ureterectomy, partial cystectomy and an extended right hemicolectomy were performed (Figure 3). A small pre-rectal lymph node was found and excised. Pathology of ureter and bladder specimen was clear cell type RCC, Fuhrman nuclear grade 4 with clear resection margin. The right hemicolectomy specimen was a moderately differentiated adenocarcinoma (pT4a pN1b). The pre-rectal lymph node was consistent with adenocarcinoma tumour deposit. Immunostaining showed the lymph node tumour cells were positive for CDX2 and negative for PAX 8, confirming it was from a colorectal primary. He refused any adjuvant therapy after discussion with oncologist.
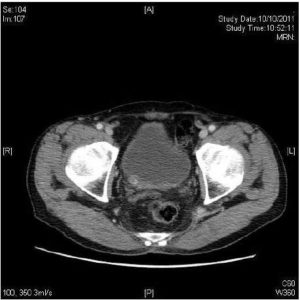
Figure 2: Suspicious Soft Tissue Mass at Right Ureteric Orifice.

Figure 3: Surgical Specimen of Completion Ureterectomy and Partial Cystectomy, with Lower as the Distal Part. (Tumour was Denoted by the Yellow Arrow)
Six months after the completion ureterectomy the patient developed haematuria again. Flexible cystoscopy revealed a necrotic tumor over the previous resected ureteric opening site. Transurethral resection of the tumour was done and pathology was clear cell type RCC. Contrast CT scan showed multiple liver metastasis. A course of haemostatic radiotherapy was completed. Total 39 Gray was given in 13 fractions, with 3 Gray per fraction and 5 fractions per week. Sunitinib 37.5mg daily was also given as target therapy. A whole body PET-CT scan and contrast CT abdomen were done one month and three months after the start of target therapy respectively. They showed progression of disease with multiple lung and liver metastases, therefore Sunitunib was stopped. Patient finally succumbed in December 2012, 29 months after the radical nephrectomy.
Discussion
Renal cell carcinoma usually metastasizes through haematogenous and lymphatic route. Frequent sites of metastasis are lung, bone and liver. It is well known for its propensity to metastasize to virtually any organs including pancreas, thyroid, skin and soft tissue. Surgical excision of RCC with solitary metastasis has been shown to improve patient’s survival. Metachronous metastasis, disease free interval of more than one year, low primary tumour stage, low tumour grade and those with lung and bone metastasis were found to have better survival after excision of solitary tumour metastasis. (Thyavihally YB et al, 2005)
RCC with ureteric metastasis is extremely rare. Only 53 cases have been reported worldwide including our case. (Chiu KY et al, 2001; Esrig D et al, 1994; Kamota S et al, 2003; Leonard AM et al, 1996; Pearce I et al, 2000; Zorn KC et al, 2006) 43 cases occurred in ipsilateral ureteric stump and 10 cases occurred in contralateral ureter. The most common presentation is recurrent haematuria after nephrectomy. (Gelister JS et al, 1992) Other presentations are ureteric obstruction and post-mortem finding. Autopsy study done by Saitoh et al found around two percent of bladder metastasis and one percent of ureteric metastasis in 1928 RCC cases. It also demonstrated an association with renal vein involvement with 6.1% of bladder metastasis in those with renal vein involvement, with only 1.2% when the renal vein is not involved. (Saitoh H, 1982)
There are three proposed mechanism of spread including haematogenous through general circulation, retrograde spread of tumour cells from renal vein or renal hilar lymphatics; direct intraluminal transit with seeding of distal urothelium. (McAchran SE et al, 2010) Haematogenous spread is supported by the synchronous discovery of widespread metastatic disease in some of the cases. Retrograde venous and lymphatic route is suggested by the preponderance of left primary renal cancer in cases of bladder metastases. Whereas the presence of tumour cells in urine substantiate tumour seeding on distal urothelium. In our case, the tumour invaded into renal calyces in the nephrectomy specimen, the proposed haematogenous spread and intraluminal transit were all possible mechanism explaining the ureteral metastases. Unfortunately, tumour recurred again in bladder after partial cystectomy. The specimen of partial cystectomy had been carefully examined and all margin was clear. The reason of the bladder recurrence could be a result of haematogenous spread, as later in the disease course patient developed wide spread liver and lung metastases.
Differentiation of clear cell RCC and clear cell transitional cell carcinoma (TCC) is not an easy task as their morphology is very similar. Sim et al performed a comparative immunohistochemical study to investigate their different characteristic. (Sim SJ et al, 1999) For example immunostaining of clear cell RCC is positive for CAM 5.2, vimentin and Leu-M1.
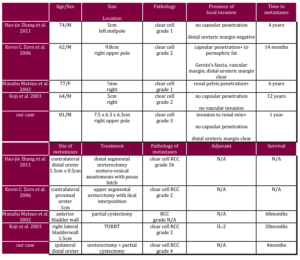
Four case reports (Matsuo M et al, 2002; Shiraishi K et al, 2003; Zhang HJ et al, 2012; Zorn KC et al, 2006) (Table 1) were studied to look into the different characteristics of ureteric metastasis. All of the cases presented as recurrent haematuria after the initial nephrectomy. Time of metastasis was very variable ranging from one year as in our case, to 12 years as in a Japanese case reported by Shiraishi et al. In all but our case the primary tumour and the metastasis were of the same Fuhrman grading. Regarding the pathology of the primary, some of these tumours had capsular penetration or renal pelvis penetration while some did not. And apparently all had a clear distal ureteric margin. All of the cases received surgical resection including transurethral resection, segmental ureterectomy or partial cystectomy. System therapy with interleukin-2 was only given in the case reported by Shiraishi K et al. In the case reported by Zorn KC et al, there was presence of focal extramedullary haematopoiesis in metastasis. The longest follow up documented was by Matsuo et al, in which the patient was free of recurrent disease at 60 months of follow up.
Table 1: Summary of Four Case Reports
References
Chiu, K. Y., Ho, H. C., Chen, J. T., Kao, Y. L., Ou, Y. C., Cheng, C. L. & Tang, C. R. (2001). “Renal Cell Carcinoma Metastasized to the Ureteral Stump,” Zhongua Xi Xue Za Zhi (Taipei); 64:64-8.
Publisher – Google Scholar
Esrig, D., Stein, J. P., Kamellos, A. W., Kiyabu, M., Freeman, J. A., & Ahlering, T. E. (1994). “Metastatic Renal Cell Carcinoma to the Contralateral Ureter,” Urology; 44:278-81.
Publisher – Google Scholar
Gelister, J. S. K., Falzon, M., Crawford, R., Chapple, C. R. & Hendry, W. F. (1992). “Urinary Tract Metastasis from Renal Carcinoma,” British Journal of Urology; 69(3):250-252.
Publisher – Google Scholar
Kamota, S., Harabayashi, T., Suzuki, S., Takeyama, Y., Mitsui, T., Mouri, G., Hashimoto, A., Nakamura, M., Shinohara, N., Nonomura, K. & Koyanagi, T. (2003). “Ureteral and Bladder Metastases of Renal Cell Carcinoma Following Synchronous Renal Cell Carcinoma and Bladder Cancer: A Case Report,” Nippon Hinyokika Gakkai Zasshi; 94:705-708.
Publisher – Google Scholar
Leonard, A. M. Y. J., Lefevre, A. & Lagrange, W. (1996). “Ureteral Stump Metastasis from Renal Adenocarcinoma: Case Report and Literature Review,” Acta Medica Belgica; 64:23-5.
Publisher – Google Scholar
Matsuo, M., Koga, S., Nishikido, M., Noguchi, M., Sakaguchi, M., Nomata, K., Maruta, N., Hayashi, T. & Kanetake, H. (2002). “Renal Cell Carcinoma with Solitary Metachronous Metastasis to the Urinary Bladder,” Urology; 60:911xiii-911xiv.
Publisher – Google Scholar
McAchran, S. E., Williams, D. H. & MacLennan, G. T. (2010). “Renal Cell Carcinoma Metastasis to the Bladder,” The Journal Urology; 184: 726-727.
Publisher – Google Scholar
Pearce, I., Harney, J. M. V. & McMahon, R. F. T. (2000). “Synchronous Presentation of Primary Renal Adenocarcinoma and Contralateral Ureteric Metastasis,” Journal of Clinical Pathology; 53:639-40.
Publisher – Google Scholar
Saitoh, H. (1982). “Distant Metastasis of Renal Adenocarcinoma in Patients with a Tumour Thrombus in the Renal Vein and/or Vena Cava,” The Journal of Urology, 127:652-653.
Publisher
Shiraishi, K., Mohri, J., Inoue, R. & Kamiryo, Y. (2003). “Metastatic Renal Cell Carcinoma to the Bladder 12 Years after Radical Nephrectomy,” International Journal of Urology; 10:453-455.
Publisher – Google Scholar
Sim, S. J., Ro, J. Y., Ordonez, N. G., Park, Y. W., Kee, K. H. & Ayala, A. G. (1999). “Metastatic Renal Cell Carcinoma to the Bladder: A Clinicopathologic and Immunohistochemical Study,” Modern Pathology; 12(4):351-355.
Publisher – Google Scholar
Thyavihally, Y. B., Mahantshetty, U., Chamarajanagar, R. S., Raibhattanavar, S. G. & Tongaonkar, H. B. (2005). “Management of Renal Cell Carcinoma with Solitary Metastasis,” World Journal of Surgical Oncology; 3:48.
Publisher – Google Scholar
Zhang, H. J., Sheng, L., Zhang, Z. W., Sun, Z. Q., Qian, W. Q. & Song, J. D. (2012). “Contralateral Ureteral Metastasis Four Years after Radical Nephrectomy,” International Journal of Surgery Case Reports, 3(1):37-38.
Publisher
Zorn, K. C., Orvieto, M. A., Mikhail, A. A., Lotan, T., Gerber, G. S., Shalhav, A. L. & Steinberg, G. D. (2006). “Solitary Ureteral Metastases of Renal Cell Carcinoma,” Urology; 68:428.e5-428.e7.
Publisher – Google Scholar






