Introduction
While the role of fungi in chronic sinusitis is well established, their contribution to the pathophysiology remains to be elucidated. Dufour, Kauffmann-Lacroix, Ferrie, et al. (2005) mentioned that fungal sinusitis (FS) has an increasing incidence in immunocompetent individuals over the last years. Aspergillus species are commonly cultured in over than 90% of the patients with chronic sinusitis, healthy individuals, or cases of fungus balls (Braun 2003, Ponikau 1999, and Willinger 2003).
The two most frequent encountered forms of FS are fungus balls and allergic fungal sinusitis (AFS). Aspergillus species are usually involved in fungus balls and are mostly related to immunosuppressed individuals (Robey et al., 2009). Mucorcolonization of the sinuses is rare and usually occurs without predisposing factors in immunocompetent individuals in contrast to mucormycosis which is the second most frequent mycosis caused by moulds in immunocompromised patients (Sugar 1992). In these patients, the species are mostly related to an acute fulminant invasive mycosis of the sinuses (Dhong 2000, Henderson 1988, and Ramadan 2006). Gamba et al. (1986) point out that paranasal sinus involvement can commence early in the course of the disease with radiological signs of mucosal thickening without formation of air-fluid level and rarely with bone erosion.
Transformation of a fungus ball of the paranasal sinus into an invasive mycosis may commence when the patient becomes immunosuppressed (Ferguson 2000). This has not been, to our knowledge, reported in the literature in association with solid tumors and oral cancer in particular. Solid tumors have been related to disseminated Mucorinfections in patients with metastases, radio or chemotherapy, but only rarely (El-Ani 1982, Yamauchi 2002).
The purpose of this effort is to present a rare case of a Mucor paranasal sinus fungus ball and its transformation into a chronic erosive noninvasive form of mycosis, possibly due to concomitant oral cancer. Diagnostic reasoning and difficulties in radiological diagnosis are being discussed.
Case Report
A 52-year-old woman was referred to our clinic, for further assessment of a squamous cell carcinoma of the tongue and floor of the mouth. The patient presented with a two-month-old swelling of the right side of the mandible.
On physical examination, a facial asymmetry caused by a right-sided facial swelling was noted. In particular, an extensive mass was palpable across the right lower border of the mandible. Intraoral examination revealed a large ulcerous lesion of the mucosa of the right side of the mandible, extending to the floor of the mouth, restricting the mobility of the tongue, and causing difficulty in swallowing.
A panoramic radiograph (Figure 1) that was obtained by a private dentist about ten months prior to consultation in order to investigate the mucosal swelling at the right mandibular alveolar ridge–then misdiagnosed as an inflammatory cyst–showed loci of calcification in the right maxillary antrum, indicative of a fungus ball.
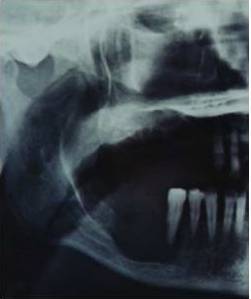
Figure 1: A Part of Panoramic Radiograph Showing a Large Osteolytic Process at the Right Side of the Mandible and Loci of Calcification in the Right Maxillary Antrum
A computed tomography (CT) scan, which was done one month before the consultation (Figure 2), demonstrated–along with a large, bone infiltrating, and contrast enhancing lesion of the floor of the mouth and the mandible–nonhomogenous micro-opacities of the right sinus with erosion and expansion of its inner wall, in whole a radiological presentation of the sinus that was mimicking malignancy.
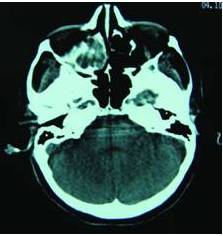
Figure 2: Axial Computed Tomography: Heterogenous Opacification of the Right Maxillary Sinus. Erosion and Expansion of the Inner Wall of the Sinus. One Month Prior to Referral
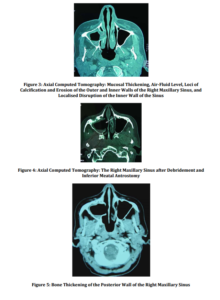
A new CT examination for staging purposes was performed at our department and revealed mucosal thickening, air-fluid level, loci of calcification, erosion of the outer and inner walls, localized disruption of the inner wall, and bone thickening of the posterior wall of the maxillary sinus (Figures 3, 4, and 5). The tumorous formation of the mandible and floor of the mouth was again demonstrated, and the infiltration of the mandible and overlying skin was noted.
The medical history of the patient contained no symptoms typical for sinus pathology, such as headache, facial or dental pain, fever, and malaise. No surgical interventions involving the maxillary sinus were reported. The patient reported no use of steroids or antibiotics during the last year.
Postnasal drip was not present. The neurological investigation revealed no abnormalities. The blood count at the day of consultation showed leukocytosis (14 X 103/μL) in particular granulocytosis (9,86 X 103/μL). Leukocytosis persisted over the last two months prior to consultation and coincided with the increase in the size of tumor growth and the infiltration of the skin.
Differential Diagnosis
Diagnostic reasoning should include other diseases of the maxillary sinus, like osteomata, carcinomata, allergic fungal sinusitis (AFS). The precence of a metal foreign body, secretions or pus should also be taken into account during the process of differential diagnosis.
The patient’s medical history contributed a lot to diagnostic reasoning. The absence of complaints like headache and mucopurulent secretions in the past strengthened the diagnosis of a nonbacteriological condition.
Foreign bodies were excluded for, according to the records of the dentist who performed the dental extractions of the maxillary teeth, all teeth or apices which were thoroughly extracted, and no tooth had undergone root canal treatment that could explain the radiopacities seen in the sinus.
Allergic fungal sinusitis was suspected since the patient’s family were farmers and the inhalation of fungal species from wheat cultivation could be a causative factor.
Blood analysis showed no eosinophilia, a finding that cannot however exclude an allergic sinusitis. No serological analysis was done to investigate the levels of IgE antibodies, because not all patients with typical signs of allergic sinusitis have increased IgE levels in their blood (Ponikau et al., 1999). Nevertheless, the absence of thick and highly viscous secretions, nasal obstruction, eosinophilic infiltration in the specimens during histological investigation, and a history of allergic rhinitis dismissed the idea of sinusitis in general and allergic sinusitis in particular. Eosinophilic infiltration is a sign of chronic rhinosinusitis independent of atopy according to Kaliner et al. (1997).
A head and neck CT scan which was performed at the day of consultation for staging purposes of the ulcerous lesion of the mandible and floor of the mouth excluded the diagnosis of an osteoma of the sinus, due to the heterogenous nonhomogenous opacification and the erosive nature of the lesion. Comparative observation of the CT obtained at our department, a CT scan from the medical record of the patient that was obtained one month before consultation and of a panoramic radiograph obtained ten months before, revealed mucosal thickening, air-fluid level, and a lesion with loci of calcification that gradually eroded the outer and inner walls of the maxillary sinus and finally disrupted the inner wall, in a whole mimicking malignancy. The observed micro-opacities could be attributed to bony remnants after bone disruption of the inner wall of the sinus or to a fungus ball and could be easily distinguished from air, chronic inflammation, polyps, and acute hemorrhage, which do appear as radiolucencies.
MRI was not used since bacterial infection, which could be better diagnosed with MRI, could be excluded from patient’s history and clinical and laboratory findings. Both bony remnants and fungus balls are indications for surgical debridement of the sinus, so it was decided to perform this procedure. The possibility of a neoplasm could not be excluded, but even if that was the case, exploring the sinus and histologically verifying the diagnosis would be the next logical step to take.
Diagnosis
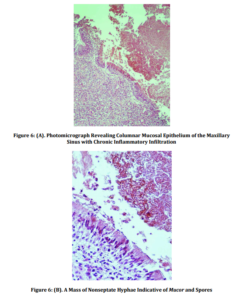
Histopathological examination revealed diffuse oedema of the sinus mucosa with inflammatory infiltration and aggregation of fibrin and polymorphonuclear leukocytes. Necrotic tissues with loci of central calcification and nonseptate hyphae, typical for Mucor species, without the presence of mycelia, mucosal involvement, or vascular invasion, were indicative of a Mucor fungus ball (Figures 6a and 6b). There were no signs of neoplasia.
Bacteriological investigation showed the presence of Staphylococcus aureus and Staphylococcus haemolyticus species normally found in chronic rhinosinusitis. No fungi could be cultured.
Management
A Caldwell-Luc operation was performed so that the sinus lesion would be further investigated. Intraoperatively, a gray discoloration of the paranasal sinus mucosa with black eschar formation was observed, while gray-black, thick, and soft fungus balls were present. The debridement was carried down to bleeding bone, while normal mucosa was spared. In the end, inferior meatal antrostomy was performed, in order to enable proper sinus drainage (Figures 4 and 5).
The patient was treated with intravenous Amphotericin B (3mg/kg/day) for ten days, under daily monitoring of the renal function.
The blood count returned to normal after sinus debridement and Amphotericin B administration, before oral cancer resection.
Two years postoperatively, the patient shows no signs of recurrence.
Discussion
According to Kaliner et al. (1997), chronic rhinosinusitis (CRS) is defined as an inflammation of the nasal and paranasal mucosa that lasts for more than 3 months. Currently, fungal colonization of the paranasal sinuses is increasingly considered in conjunction with CRS. The first to describe fungal sinusitis were Katzenstein et al. (1983), who termed the condition allergic Aspergillus sinusitis. It was considered of allergic nature as it presented common histopathological characteristics with allergic bronchopulmonary aspergillosis (ABPA). Ferguson (2000) mentions that no relation has been established between fungal sinusitis and previous sinus pathology. Likewise, our patient’s medical record did not reveal a history of sinusitis. The exact role of fungi in the natural history of chronic rhinosinusitis remains unclear.
Sinus mycoses have been classified into four types by Katzenstein et al. (1983): (1) noninvasive chronic or fungus ball, (2) allergic, (3) chronic indolent invasive, and (4) fulminating invasive mycoses. Fungus balls may also appear in association with allergic mycoses, with symptoms other than those of chronic sinusitis (de Shazo et al., 1997). A condition of intermediate severity between a fungus ball and a chronic invasive mycosis is the chronic noninvasive erosive mycosis (Uri et al., 2003), which was observed in our patient. This is a fungus ball with no tissue invasion but bone erosion and extensive disease that is linked, according to Rowe-Jones and Moore-Gillon (1994) and Panda et al. (2004), most of the times to Aspergillus species.
For the diagnosis of the fungus ball, we used the criteria posed by de Shazo et al. (1997): (1) partial sinus opacification with calcification; (2) clay-like material within the sinus; (3) conglomeration of hyphae not involving sinus mucosa; (4) chronic inflammatory infiltration; (5) no predominance of eosinophils, no granuloma, and no allergic mucin; (6) no invasion of bone, vessels, or mucosa.
Cultures from fungus balls mostly reveal Aspergillus species (Braun 2003, Ponikau 1999, and Willinger 2003). In our case, the colonization was dominated by Mucor species, which do rarely form fungus balls (Table 1) and are mostly related to an acute fulminant invasive mycosis of the sinuses (Dhong 2000, Goodnight 1993, Henderson 1988, Pérez Fernández 2001, Ramadan 2006, and Saydam 1997).
Table 1: Cases of Mucor Fungus Balls of the Paranasal Sinuses
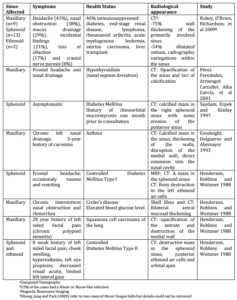
A fungus ball appears usually in one sinus, most frequently the maxillary, and is noninvasive. A rare case of an involvement of more than one sinus was presented by Chao (2004).
They develop in immunocompetent individuals with no predisposing factors, although local tissue hypoxia, massive fungal exposure, a positive airway pressure apparatus, dental treatment, nasal septum deviation, and osteomeatal complex occlusion may be causally related (Barry 2002, Corey 1990, Henderson 1988, and Pérez Fernández 2001).
A fungus ball may become invasive when the patient becomes immunosuppressed (Ferguson 2000 and Sugar 1992). Our patient had no leukopenia, thus she could not be considered immunosuppressed. Additionally, no predisposing factors like steroid uptake, diabetes mellitus, or nasal septum deviation that could explain the transformation could be evaluated (Pérez Fernández et al., 2001). However, oral cancer should be approached as a condition of immune system dysregulation and impairment, giving a possible explanation for the transformation of the fungus ball into an erosive form of mycosis.
The diagnosis of the species involved in fungal sinusitis is set with histopathological examination of biopsy samples of tissue nearby necrotic areas, stained with hematoxylin-eosin or special stain, like silver methenamine or periodic acid Schiff (PAS) (Kostamo et al., 2004). Cultures often fail to detect Mucor species, because fungi are very often degenerating and the mucus is viscous, inhibiting the proper contact of the species with the growth medium (Braun 2003 and Katzenstein 1983). Molecular techniques, like DNA sequencing and hybridization, offer the opportunity to detect fungi in fungus balls (Robey et al., 2009).
Kostamo et al. (2004) highlight that the detection of fungi in chronic sinusitis cannot prove evidence of the fact that they are responsible for the pathology, but it supports the opinion that the switch in the microflora is related to the chronic course of the disease. Conclusions regarding the aggressiveness of the condition cannot be drawn according to the organisms encountered during laboratory investigations according to Zinreich et al. (1988).
Plain radiographs may present loci of hyperattenuation (Table 2) in the paranasal sinuses, which are due to the presence of calcium phosphate and sulfate in the center of fungal masses (Stammberger et al., 1983). Signs typical of fungal sinusitis, like mucoperiosteal thickening, wall destruction, and increased attenuation, may also represent neoplasia (Zinreich et al., 1988). Thus, plain radiographs are nonspecific for the diagnosis of fungal sinusitis.
Table 2: Radiographical Investigation of Fungus Balls of the Maxillary Sinus: Appearance and Differential Diagnosis (Castelnuovo 2000, Corey 1995, Goodnight 1993, Som 1990)
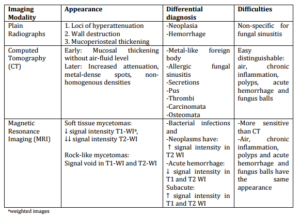
Computed tomography (CT) examination of paranasal sinus fungus balls early in the course of the infection demonstrates mucosal thickening without formation of air-fluid level (Goodnight et al., 1993). Later in the progression of the disease, CT usually shows areas of nonhomogenous intensity or metal-like foreign body (Castelnuovo et al., 2000). Increased attenuation in masses within the sinus suggests a fungal involvement, in particular the presence of Aspergillus(Zinreich et al., 1988). A popcorn appearance was also described (Goodnight et al., 1993). This heterogenous densities can also be indicative of AFS, thick mucoid secretions, pus, thrombi, or even carcinomata and osteomata (Zinreich 1988, Corey 1995).
Magnetic resonance imaging (MRI) in case of a fungal infection shows a slightly decreased intensity in T1-weighted images and a considerably decreased intensity in T2-weighted images, in contrast to bacterial infections and neoplasms where there is an increased signal intensity in T2-weighted images (Corey 1995, Zinreich 1988). This is true for soft fungus balls, whereas rock-like fungus balls produce signal void on all images (Goodnight et al., 1993). These characteristics are thought to be due to higher levels of magnesium, manganese, iron, essential for fungal metabolism, calcium in the presence of fungi, air, and varying degrees of dehydration (Dedyukhina 1991, Goodnight 1993, Som 1990, and Schwartz 1992).
Because of the similarity to fungus balls high attenuation of hemorrhage in CT images, the last is being distinguished from fungus balls with the help of MRI; a hemorrhage, if subacute, has increased signal intensity both in T1- and T2-weighted images, if acute a decreased signal intensity (Zinreich et al., 1988).
According to Broglie et al., CT imaging has a positive prognostic value of 56% and a negative prognostic value of 98% in diagnosing paranasal sinus fungus balls (Broglie et al., 2009). Thus, it fails to detect about half of the patients with the condition. A similarly low sensitivity of 62% was reported by Dhong et al. (2000). On the other hand, when the diagnostic criterion is high attenuation, CT examination detects 75% of the patients with fungal sinusitis (Zinreich et al., 1988).
MRI is more sensitive in diagnosing fungal sinusitis (Zinreich et al., 1988). There is a problem behind the issue of the superiority of one modality over the other. In particular, there is no clear superiority of MRI compared to CT, because materials such as air, chronic inflammation, polyps, and acute hemorrhage have the same presentation with fungus balls on MRI. Thus, a fungus ball can be with difficulty distinguished from air and acute hemorrhage, whereas air can be misinterpreted as fungus ball or chronic inflammation (Som et al., 1990). MRI is a useful tool in case of pansinusitis or involvement of the sphenoid sinus (Dufour et al., 2005).
Bone erosion is less frequently reported in relation to fungal sinusitis (4%—17%) (Rowe-Jones and Moore-Gillon 1994). It should not be taken as a sign of invasive disease in the absence of histopathological findings of invasion according to Corey et al. (1995) and Handley et al. (1990). Possible etiological factors of bone erosion are enzymes produced by the fungus ball, inflammatory mediators resulting from atopy and pressure (Corey et al. 1995). Tovi et al. (1992) stress that bone thickening and sclerosis of the walls of paranasal sinuses are related to chronic or recurrent sinus inflammation and result from osteoblastic osteitis. According to the previously mentioned authors, osteoblastic and osteolytic changes may appear simultaneously, creating a mixed radiological image. Braun and Bourjat (2000) highlight that bone changes should not be taken as a sign of chronicity. Both bone erosion and thickening were present in our case.
The present report is unique in that it presents a rare case of a paranasal sinus fungus ball by genera of the Mucoraceae family, of the order Mucorales and its transformation into a chronic erosive noninvasive form of mycosis, possibly due to concomitant oral cancer. Mucor fungus balls have not been previously associated with oral cancer.
We suggest that clinicians should include Mucor colonization in their differential diagnosis in patients, compromised or not, with sinusitis. The diagnosis of paranasal sinus mycoses, and the distinction from other pathologic conditions affecting the maxillary sinus , necessitates a proper radiological investigation in addition to a thorough clinical examination and a good medical history.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
Barry, B., Topeza, M. & Géhanno, P. (2002). “Aspergillosis of the Paranasal Sinus and Environmental Factors,” Annales D’ Oto-Laryngologie et de Chirurgie Cervico-Faciale, 119 (3) 170-173.
Publisher – Google Scholar
Braun, H., Buzina, W., Freudenschuss, K., Beham, A. & Stammberger, H. (2003). “Eosinophilic Fungal Rhinosinusitis: A Common Disorder in Europe?,” Laryngoscope, 113 (2) 264-269.
Publisher – Google Scholar
Braun, J. J. & Bourjat, P. (2000). “CT Imaging of Fungal and Nonfungal Caseous Sinusitis. A Report of 50 Cases,”Journal de Radiologie, 81 (3) 227-231.
Publisher – Google Scholar
Broglie, M. A., Tinguely, M. & Holzman, D. (2009). “How to Diagnose Sinus Fungus Balls in the Paranasal Sinus? An Analysis of an Institution’s Cases from January 1999 to December 2006,” Rhinology, 47 (1) 379-384.
Publisher – Google Scholar
Castelnuovo, P., Gera, R., Di Giulio, G., Canevari, F. R., Benazzo, M., Emanuelli, E., Galli, J., Di Girolamo, S. & Staffieri, A. (2000). “Paranasal Sinus Mycoses,” Acta Otorhinolaryngologica Italica, 20 (1) 6-15.
Publisher – Google Scholar
Chao, T. K. (2004). “Triple Discrete Fungus Balls of the Paranasal Sinuses,” Otolaryngology– Head and Neck Surgery,131 (6) 1014-1015.
Publisher – Google Scholar
Corey, J. P., Delsupehe, K. G. & Ferguson, B. J. (1995). “Allergic Fungal Sinusitis: Allergic, Infectious, or Both?,”Otolaryngology– Head and Neck Surgery, 113 (1) 110-119.
Publisher – Google Scholar
Corey, J. P., Romberger, C. F. & Shaw, G. Y. (1990). “Fungal Diseases of the Sinuses,” Otolaryngology– Head and Neck Surgery, 103 (6) 1012-1015.
Publisher – Google Scholar
Dedyukhina, E. G. & Eroshin, V. K. (1991). “Essential Metal Ions in the Control of Microbial Metabolism,” Process Biochemistry, 26 (1) 31—37.
Publisher – Google Scholar
De Shazo, R. D., O’Brien, M., Chapin, K., Soto-Aguilar, M., Swain, R., Lyons, M., Bryars, W.C. Jr & Alsip, S. (1997). “Criteria for the Diagnosis of Sinus Mycetoma,” The Journal of Allergy and Clinical Immunology, 99 (4) 475—485.
Publisher – Google Scholar
Dhong, H. J., Jung, J. Y. & Park, J. H. (2000). “Diagnostic Accuracy in Sinus Fungus Balls: CT scan and Operative Findings,” American Journal of Rhinology, 14 (4) 227—231.
Publisher – Google Scholar
Dufour, X., Kauffmann-Lacroix, C., Ferrie, J. C., Goujon, J. M., Rodier, M. H., Karkas, A. & Klossek, J. M. (2005). “Paranasal Sinus Fungus Ball and Surgery: A Review of 175 Cases,” Rhinology, 43 (1) 34-39.
Publisher – Google Scholar
El-Ani, A. S. & Dhar, V. (1982). “Disseminated Mucormycosis in a Case of Metastatic Carcinoma,” American Journal of Clinical Pathology, 77 (1) 110-114.
Publisher – Google Scholar
Ferguson, B. J. (2000). “Fungus Balls of the Paranasal Sinuses,” Otolaryngologic Clinics of North America, 33 (2) 389-398.
Publisher – Google Scholar
Gamba, J. L., Woodruff, W. W., Djang, W. T. & Yeates, A. E. (1986). “Craniofacial Mucormycosis: Assessment with CT,” Radiology, 160 (1) 207-212.
Publisher – Google Scholar
Goodnight, J., Dulguerov, P. & Abemayor, E. (1993). “Calcified Mucor Fungus Ball of the Maxillary Sinus,” American Journal of Otolaryngology, 14 (3) 209-210.
Publisher – Google Scholar
Handley, G. H., Visscher, D. W. & Katzenstein, A. A. (1990). “Bone Erosion in Allergic Fungal Sinusitis,” American Journal of Rhinology, 4 (4) 149-153.
Publisher – Google Scholar
Henderson, L. T., Robbins, K. T., Weitzner, S., Dyer, T. C. & Jahrsdoerfer, R. A. (1988). “Benign Mucor Colonization (Fungus Ball) Associated with Chronic Sinusitis,” Southern Medical Journal, 81 (7) 846-850.
Publisher – Google Scholar
Kaliner, M. A., Osguthorpe, J. D., Fireman, P., Anon, J., Georgitis, J. & Davis, M. L. (1997). “Sinusitis: Bench to Bedside–Current Findings, Future Directions,” Otolaryngology– Head and Neck Surgery, 117 (6) 1—20.
Publisher – Google Scholar
Kaplan, A. L., Huerta, A. R. & Chiou, S. J. (1981). “Rhinocerebral Mucormycosis,” The Western Journal of Medicine,135 (4) 326-329.
Publisher
Katzenstein, A. L. A., Sale, S. R. & Greenberger, P. A. (1983). “Allergic Aspergillus Sinusitis: A Newly Recognized form of Sinusitis,” The Journal of Allergy and Clinical Immunology, 72 (1) 89—93.
Publisher – Google Scholar
Kostamo, K., Richardson, M., Virolainen-Julkunen, A., Leivo, I., Malmberg, H., Ylikoski, J. & Toskala, E. (2004). “Microbiology of Chronic Hyperplastic Sinusitis,” Rhinology, 42 (4) 213-218.
Publisher – Google Scholar
Panda, N. K., Balaji, P., Chakrabarti, A., Sharma, S. C. & Reddy, C. E. (2004). “Paranasal Sinus Aspergillosis: Its Categorization to Develop a Treatment Protocol,” Mycoses, 47 (7) 277—283.
Publisher – Google Scholar
Pérez Fernández, C. A., Armengot Carceller, M., Alba García, J. R., Montero Balaguer, B., Ballester, E. & Basterra Alegría, J. (2001). “Benign Sinusal Mucor Colonization in Association with Septal Deviation,” Acta Otorrinolaringologica Espanola, 52 (2) 157-161.
Publisher – Google Scholar
Ponikau, J. U., Sherris, D. A., Kern, E. B., Homburger, H. A., Frigas, E., Gaffey, T. A. & Roberts, G. D. (1999). “The Diagnosis and Incidence of Allergic Fungal Sinusitis,” Mayo Clinic Proceedings, 74 (9) 877—884.
Publisher – Google Scholar
Ramadan, H. H. (2006). “Chronic Rhinosinusitis and Bacterial Biofilms,” Current Opinion in Otolaryngology & Head and Neck Surgery, 14 (3) 183-186.
Publisher – Google Scholar
Robey, A. B., O’Brien, E. K., Richardson, B. E., Baker, J. J., Poage, D. P. & Leopold, D. A. (2009). “The Changing Face of Paranasal Sinus Fungus Balls,” The Annals of Otology, Rhinology, and Laryngology, 118 (7) 500-505.
Publisher – Google Scholar
Rowe-Jones, J. M. & Moore-Gillon, V. (1994). “Destructive Noninvasive Paranasal Sinus Aspergillosis: Component of a Spectrum of Disease,” The Journal of Otolaryngology, 23 (2) 92-96.
Publisher – Google Scholar
Saydam, L., Erpek, G. & Kizilay, A. (1997). “Calcified Mucor Fungus Ball of Sphenoid Sinus: An Unusual Presentation of Sinoorbital Mucormycosis,” The Annals of Otology, Rhinology, and Laryngology, 106 (10) 875-877.
Publisher – Google Scholar
Schwartz, H. J., Witt, W. J. & Sher, T. H. (1992). “Allergic Bronchopulmonary Aspergillosis and Allergic Aspergillus Sinusitis: Case Report,” Annals of Allergy, 69 (5) 447-448.
Publisher – Google Scholar
Som, P. M., Dillon, W. P., Curtin, H. D., Fullerton, G. D. & Lidov, M. (1990). “Hypointense Paranasal Sinus Foci: Differential Diagnosis with MR Imaging and Relation to CT Findings,” Radiology, 176 (3) 777-781.
Publisher – Google Scholar
Stammberger, H., Jakse, R. & Raber, J. (1983). “Aspergillus-Mykosen der Nasennebenhöhlen. Nachweis und Analyse röntgendichter Strukturen im Pilzkonkrement,” Hals-Nasen- Ohren Heilkunde, 31 (5) 161-167.
Publisher
Sugar, A. M. (1992). “Mucormycosis,” Clinical Infectious Diseases, 14 (Suppl 1) 126-129.
Publisher – Google Scholar
Tovi, F., Benharroch, D., Gatot, A. & Hertzanu, Y. (1992). “Osteoblastic Osteitis of the Maxillary Sinus,” The Laryngoscope, 102 (4) 426-430.
Publisher – Google Scholar
Uri, N., Cohen-Kerem, R., Elmalah, I., Doweck, I. & Greenberg, E. (2003). “Classification of Fungal Sinusitis in Immunocompetent Patients,” Otolaryngology– Head and Neck Surgery, 129 (4) 372-378.
Publisher – Google Scholar
Willinger, B., Obradovic, A., Selitsch, B., Beck-Mannagetta, J., Buzina, W., Braun, H., Apfalter, P., Hirschl, A. M., Makristathis, A. & Rotter, M. (2003). “Detection and Identification of Fungi from Fungus Balls of the Maxillary Sinus by Molecular Techniques,” Journal of Clinical Microbiology, 41 (2) 581-585.
Publisher – Google Scholar
Yamauchi, T., Misaki, H., Arai, H., Iwasaki, H., Naiki, H. & Ueda, T. (2002). “An Autopsy Case of Disseminated Mucormycosis in a Neutropenic Patient Receiving Chemotherapy for the Underlying Solid Malignancy,” Journal of Infection and Chemotherapy, 8 (1) 103-105.
Publisher – Google Scholar
Zinreich, S. J., Kennedy, D. W., Malat, J., Curtin, H. D., Epstein, J. I., Huff, L. C., Kumar, A. J., Johns, M. E. & Rosenbaum, A. E. (1988). “Fungal Sinusitis: Diagnosis with CT and MR Imaging,” Radiology, 169 (2) 439-444.
Publisher – Google Scholar








