Introduction
Semmens, (1962) reported the incidence of congenital Müllerian anomalies ranged from 1:500 to 1:2000. Simon et al (1991) claimed that the incidence was as high as 3.2%. A unicornuate uterus results from the partial or complete lack of development of one Müllerian duct during the first 7—8 weeks of embryonic life. Raga et al, (1997) found the incidence of unicornuate uterus in a retrospective longitudinal review of 3181 patients of reproductive age desiring to conceive, was 0.06%. Heinonen (1997) contends that about 5-10% of all uterine anomalies are of the unicornuate variety most of which contain rudimentary horns with or without cavitation.
The rudimentary horn may be cavitated with endometrium or non-cavitated, and may or may not communicate with the functioning unicornuate uterus (Falcone et al., 1997). An estimated 75—90% of unicornuate uteri with rudimentary horns are non-communicating (O’Leary and O’Leary, 1963; Buttram and Gibbons, 1979; Rock and Schlaff, 1985).
In the case of a blind non-communicating, cavitated rudimentary horn with a functioning endometrium, cryptomenorrhoea may lead to dysmenorrhoea soon after menarche and result in haematometra. Olive and Henderson, (1987) believe that retrograde menstruation from a functioning rudimentary horn through a patent ipsilateral Fallopian tube may result in the development of haematosalpinx and endometriosis. Dicker et al, (1998), argues that a rudimentary horn pregnancy can result from transperitoneal migration of spermatozoa.
We present a case of a term pregnancy in a patient with a unicornuate uterus and in whom the only ovarian tissue was attached to the right rudimentary horn. We believe that this is the best evidence produced for transperitoneal migration of an oocyte and it is the first case of a naturally conceived term pregnancy resulting from transperitoneal migration of an ovum in the presence of a mullerian duct abnormality.
Case Report
A 40-year —old Gravida 2 para 1 was admitted for an elective caesarean section, the indication for the procedure was a breech presentation at term in whom an external cephalic version was attempted but failed.
This pregnancy was naturally conceived after eleven years of regular unprotected coitus. Her antenatal period was uneventful and her first and second trimester ultrasound scans did not reveal any uterine abnormality.
An ultrasound scan at 37 completed weeks of gestation confirmed the clinical suspicion that the foetus was presenting by the breech. Options for her care were discussed and an external cephalic version was attempted with the patient’s consent. The external cephalic version failed and the patient was scheduled for an elective caesarean section at 39 completed weeks gestation.
Thirteen years earlier, the patient had an assisted vaginal delivery of a 3kg female infant who has been well. Three years prior to conceiving, the patient underwent a laparotomy and left oophorectomy for an 11cm benign left ovarian tumour. At surgery, the patient’s uterine abnormality was not recognised. Endometriosis was noted on the left ovarian cyst wall, the uterovesical fold and in the left ovarian fossa. She had regular monthly menstrual cycles with no history of dysmenorrhoea.
Casarean Section
On completion of 39 weeks gestation, the patient underwent an elective caesarean section. A live male of birth weight 3.17kg was delivered from a left unicornuate uterus to which was attached, a left fallopian tube, no left ovary and a right sided uterine horn bearing a normal looking ovary and tube (see figures 1,2 and 3).
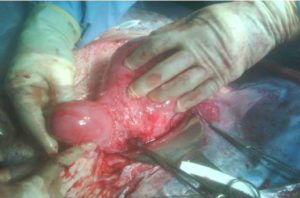
Figure 1: Right Rudimentary Horn
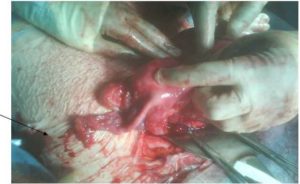
Figure 2: Right Rudimentary Horn Attached With the Right Tube and Ovary
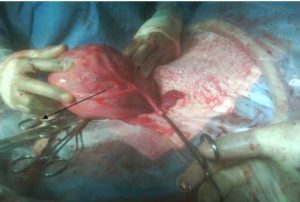
Figure 3: Unicornuate Uterus with Left Fallopian Tube and Absent Ovary
The absent left ovary was in keeping with the patient’s history of a previous left oophorectomy. Mild endometriosis was noted on the uterovesical fold, ventral surface of the uterus and in the right ovarian fossa. No other abnormalities were identified during the surgery. A single cervix was identified. The patient’s post-operative course was uneventful.
Imaging studies did not reveal any evidence of renal nor urinary tract abnormality. Additionally, there was no evidence of communication between the rudimentary horn and the unicornuate uterus on hysterosalpingogram.
Discussion
Unicornuate uterus with a rudimentary horn is a rare type of mullerian duct malformation and results from the defective fusion of the malformed duct with the contra-lateral duct (Crosby 1962). The rudimentary horn may consist of a functional endometrial cavity or it may be a small solid lump of uterine muscle with no functional endometrium.
Imaging studies in our patient did not reveal any functional endometrium in the non-communicating horn and as such our patient, as expected, did not suffer from symptoms of obstruction to menstrual flow in the non-communicating horn.
We believe that this patient’s pregnancy could only have resulted from the transperitoneal migration of the ovum produced by her right ovary which was attached to the non-communicating right uterine horn. Since this was the only ovarian tissue identified in this patient, it would further suggest that once the ovum was released from the right ovary it was picked up by the left fallopian tube.
We further submit that an ovum could have only been attracted to and taken up by the left tube by factors other than the proximity of the tube to the ovary. The geographical proximity of the right tube to the ovum would seem the more plausible expectation, since the prevailing view suggest that just prior to ovulation the fimbriae of the infundibulum place themselves around the ovarian stigma and seals off the location. Via rotation about the axis of the suspensory ligament of the ovary and the ovarian ligament, the ovary can turn the follicle that is about to rupture towards the fallopian tube. Hence it facilitates the uptake of the released ovum.
What could this factor other than the geographical proximity be? Could the semen from the male partner play a role? Likely, if fertilisation takes place in the fallopian tube and a mature ovum is fertilizable for up to 24 hours after release but maximally fertilizable for only 8 to 12 hours and the human sperm can remain viable in the female genital tract for 24 to 72 hours but can remain healthy and highly fertile probably for only 12 to 24 hours, it would seem probable that the semen available in the reproductive tract could release factors to attract the ovum. Since the right uterine horn did not communicate with the left horn, it would not be expected to have any semen. Thus if the chemotactic factors are produced by the semen, the ovum would not be expected to be attracted to the right horn.
Could the tubes themselves be responsible for the release of these chemotactic factors? Highly unlikely since the preference of the ovum for the more distant left tube argues strongly against the tube as the source of this chemotactic factor.
Could the endometrium be involved in the release of these chemotactic factors? Possibly, there was no discernible functional endometrium in the right uterine horn. The endometrium of the unicornuate uterus could therefore also release these factors to attract the ovum to the tube and into the unicornuate uterus.
Alternatively, both the endometrium and semen could play additive roles in the release of these factors to attract the ovum. Physiologically, the endometrium could be the more plausible source, since it plays a role in the nidation and early embryonic development, whereas semen does not seem to play any such role.
In an effort to support our premise, we performed an English language search of the literature following data bases Pubmed, Embase, Cinahl and Medline using search terms Human Pregnancy, one ovary, one fallopian tube, we recovered 82 reports. Our search was then refined to reflect contralateral fallopian tube, and we recovered only 4 reports. In a case series of one hundred and fourteen cases of ectopic pregnancies, Walter’s et al (1987) found that in 16% of cases in his series, the corpus luteum was on the contralateral side. Wheeler (1985) contends that transperitoneal migration of ova resulting in an intrauterine pregnancy is probably a common event that is rarely observed and Honore´ in 1978 reported on five cases of ectopic pregnancies with contralateral corpus luteum.
One could argue that the sperm could exit the left fallopian tube and through the transperitoneal sperm migration as proposed by Nahum et al (2004) fertilize the ovum in the peritoneal cavity and then the fertilised ovum migrated to the uterine cavity via the other tube. This would be most unlikely in our case since there was no communication between the right horn and the cavity of the left uterus.
Our literature search in databases including Medline, Cinahl, Embase and Pubmed (English language, search terms: Transperitoneal migration, unicornuate uterus, Mullerian duct abnormality, term pregnancy) failed to retrieve similar case reports. We therefore propose that this is the first natural experiment which has provided indisputable evidence of a naturally conceived term pregnancy in a unicornuate uterus resulting from transperitoneal ovum migration.
Weaknesses
We recognise that there are a number of difficulties with our case study, among them are (a) the failure to recognise the uterine abnormality at the time of the laparotomy and left oophorectomy, (b) The attempted external cephalic version in the presence of a uterine abnormality, though at the time of the attempt, it was not known that this patient had a uterine abnormality, (c) The failure to excise the uterine horn when it was identified at the caesarean section; it must however be understood that this was an unexpected finding and the patient’s signed consent did not allow us to perform this procedure and (d) since this is only a case report, it suffers from the same general weaknesses of case reports and thereby limits the conclusions which can be drawn. However, we believe that if one is able to look past these weaknesses, a number of lessons can be learnt.
Conclusion
Several lessons can be learnt from this natural experiment. It provides the strongest evidence to date of transperitoneal migration of an ovum. It tacitly argues for the role of chemotactic factors in the movement of an ovum to the fallopian tube after ovulation, and simultaneously suggests that the geographical proximity of the fallopian tube to the ovary is not the only factor involved in the uptake of the released ovum. Whether this chemotactic factor plays a role in the absence of a uterine abnormality or whether it is only manifested when geographical proximity fails to yield a pregnancy remains unanswered. It may well be that chemotactic factors are always present but plays a secondary role in the presence of normal anatomy but as long as the normal anatomy is disturbed the chemotactic factors seem to gain ascendancy in their role of facilitating tubal uptake of a released ovum.
References
Buttram, V. C. Jr, Gomel, V., Siegler, A., Decheney, A., Gibbons, W. & March, C. (1988). “The American Fertility Society Classification of Adnexal Adhesions, Distal Tubular Occlusions, Tubal Occlusions Secondary to Tubal Ligation, Tubal Pregnancies, Mullerian Anomalies and Intrauterine Adhesions,” Fertil Steril, 49 944-955.
Publisher
Crosby, W. M. & Hill, E. C. (1962). “Embryology of the Mullerian Duct System. Review of Present Day Theory,”Obstet Gynecol, 20 507-515.
Publisher – Google Scholar
Dicker, D., Goldman, J. A. Ashkenazi, J. Feldberg, D. & Deliel, A. (1990). “The Value of Hysteroscopy in Elderly Women Prior to in Vitro Fertilisation-Embryo Transfer (IVF-ET): A Comparative Study,” J In Vitro Fert Embryo Transf, 7 267-270.
Publisher – Google Scholar
Falcone, T., Gidwani, G., Paraiso, M., Beverly, C. & Goldberg, J. (1997). “Anatomical Variation in the Rudimentary Horns of a Unicornuate Uterus, Implications for Laparoscopic Surgery,” Hum Rep, 12 263-265.
Publisher – Google Scholar – British Library Direct
Grimbizis, G. F., Camus, M., Tarlatzis, B. C., Bontis, J. N. & Devroey, P. (2001). “Clinical Implications of Uterine Malformations and Hysteroscopic Treatment Results,” Hum Reprod Update, 7 161-74.
Publisher – Google Scholar – British Library Direct
Heinonen, P. K. (1997). “Reproductive Performance of Women with Uterine Anomalies after Abdominal or Hysteroscopic Metroplasty or No Surgical Treatment,” J. Am Assoc Gynecol Lapaosc, 4 311-317.
Publisher – Google Scholar – British Library Direct
Honore, L. H. (1978). “Tubal Ectopic Pregnancy with Contralateral Corpus Luteum: A Report of Five Cases,” J. Reprod Med, 21 (4) 269-271.
Publisher – Google Scholar
Jayasinghe, Y., Rene, A., Stalewski, H. & Gravien, S. (2005). “The Presentation and Early Diagnosis of the Rudimentary Uterine Horn,” Obstet Gynecol, 105 1456-67.
Publisher – Google Scholar
Nahum, G. G., Stanislaw, H. & Mcmahon, C. (2004). “Preventing Ectopic Pregnancies: How Often Does Transperitoneal Transmigration of Sperm Occur in Effecting Human Pregnancy?,” BJOG: An International Journal of Obstetrics and Gynaecology, 111(7) 706-714.
Publisher – Google Scholar
O’Leary, J. L. & O’Leary, J. A. (1963). “Rudimentary Horn Pregnancy,” Obstet. Gynecol, 22 371 -375.
Publisher – Google Scholar
Olive, D. L. & Henderson, D. Y. (1987). “Endometriosis and Mullerian Anomalies,” Obstet Gynecol, 69 (3pt1) 412-415.
Publisher – Google Scholar
Raja, F., Bauset, C., Remoli, J., Bonilla-Musoles, F., Simón, C. & Pellicen, A. (1997). “Reproductive Impact of Congenital Mullerian Anomalies,” Hum Reprod, 12(10) 2277-2281.
Publisher – Google Scholar – British Library Direct
Rock, J. A. & Schlaff, W. D. (1985). “The Obstetric Consequences of Uterovaginal Anomalies,” Fertil Steril, 43 681-692.
Publisher
Semmens, J. P. (1962). “Congenital Anomalies of Female Genital Tract: Functional Classification Based on Review of 56 Personal Cases and 500 Reported Cases,” Obstet Gynecol, 19:328.
Publisher – Google Scholar
Simón, C., Martinez, L. Pardo, F. Tortojada, M. & Pellicen, A. (1991). “Mullerian Defect in Women with Normal Reproductive Outcome,” Fertil Steril, 56(6) 1192-3.
Publisher
Tufail, A. & Hashmir, H. A. (2007). “Ruptured Ectopic Pregnancy in Rudimentary Horn of the Uterus,” JCPSP 17(2) 105-106.
Publisher – Google Scholar
Walters, M. D., Eddy, C. & Pauerstein, C. J. (1987). “The Contralateral Corpus Luteum and Tubal Pregnancy,” Obstet Gynecol, 70 (6) 823-826.
Publisher
Wheeler, J. M. & Dodson, M. G. (1985). “Transperitoneal Migration of the Ovum. A Case Report,” J. Reprod Med, 30 (11) 895-898.
Publisher – Google Scholar





