Introduction
Large pituitary adenomas with moderately high prolactin (PRL) levels are a diagnostic challenge. It is often difficult to distinguish between prolactinoma and nonfunctioning tumour, especially when PRL values fall within the so called “grey zone”. The latter includes serum PRL concentrations ranging between 100 and 200 ng/mL. PRL values above 200 ng/mL are compatible with a diagnosis of a macroprolactinoma. However, the non-defining PRL levels of the “grey zone” could be justified by a nonfunctioning adenoma that is displacing the pituitary stalk (Karavitaki, et al., 2006). According to Pituitary Society guidelines (2006), surgical treatment is the elective treatment in the case of a nonfunctioning tumour. In contrast, pharmacologic treatment with dopamine agonists is the first option considered whenever a prolactinoma is suspected. The response of nonfunctioning tumours to dopamine agonists treatment has been documented scarcely (Bevan, Webster, Burke and Scanlon, 1992; Masding, Lees, Gawne-Cain and Sandeman, 2003). Nevertheless, this response is plausible due to the frequent existence of dopamine D2 subtype receptors in nonfunctioning adenoma cells (Ivan, et al., 2005).
The response of a prolactinoma to dopamine agonist treatment is obtained in the majority of patients. Molitch, et al. (1985) described serum PRL levels’ normalization and restoration of the menstrual cycle in 70-80% of the cases. Tumour shrinkage was observed in around 70% of the cases. Maintenance of treatment for 24 months or longer is required in order to prevent tumoural re-growth. In fact, macroprolactinoma enlargement is expected to occur several weeks after discontinuation of even a long-term therapy (Johnston, et al., 1984). Besides, a prolactinoma could re-grow during pregnancy due to hyperplasia of pituitary lactotroph cells (Bronstein, Salgado and de Castro Musolino, 2002; Molitch, 2003). Women should be informed about the risk and said that their fertility may be restored once hyperprolactinaemia has been corrected. Pregnancy induces the tumour re-growth frequently, particularly in patients harbouring macroprolactinomas, with a subsequent risk of neurological invasion (Foyouzi, Frisbaek and Norwitz, 2004).
A woman with a pituitary macroadenoma and hyperprolactinaemia attended our clinics. Based on a possible diagnosis of macroprolactinoma, she was treated with the dopamine agonist Bromocriptine showing a substantial reduction of the tumour. This reduction was maintained despite both an early withdrawal of the drug and an incident pregnancy. In retrospect, these findings suggest the diagnosis of a nonfunctioning adenoma that had a marked decrease in size after it was treated with Bromocriptine. This unusually good response was not expected in a non-Functioning adenoma.
Case Report
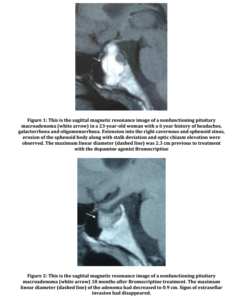
A 23-year-old woman was admitted to hospital with headache, galactorrhoea and oligomenorrhea for six years. No other past medical history was relevant for the case. Temporal hemianopsia in the left eye was revealed by a perimetry test. Laboratory tests showed a serum prolactin level of 147.0 ng/mL (normal 0.0-18.3 ng/mL) measured by a chemiluminescent assay (Elecsys from Roche, Mannheim, Germany), with a peak of 177 ng/mL after stimulating with thyrotropin releasing hormone. Secondary causes of hyperprolactinaemia were ruled out: concomitant use of any drugs or toxic substances, the existence of herpetic or traumatic thoracic lesions, thyroid, adrenal, hepatic or renal dysfunction and the presence of polycystic ovary syndrome. Acromegalic features were not observed and insulin growth factor-1 levels were within normal values for age (obtained by the chemiluminescent immunometric assay Immulite 2500, Siemens, Erlangen, Germany). The hypophyseal magnetic resonance imaging (Signa 1.5 tesla, General Electric Healthcare, Milwaukee, Wisconsin, United States) showed a lobulated pituitary mass extending into the right cavernous and sphenoid sinus with erosion of the sphenoid body, displacing the pituitary stalk and the optic chiasm. The maximum linear diameter of the lesion was 2.3 cm (Fig. 1).
A modest hyperprolactinaemia could be caused by a PRL-secreting tumour. However, PRL levels are typically higher than the above described when encountered as a result of large macroprolactinomas. Alternatively, this woman hyperprolactinemia could be a consequence of a compressive effect exerted by a nonfunctioning pituitary tumour on the hypophyseal stalk. The treatment of these possibilities differs. Pharmacologic treatment with a dopamine agonist is the first line option whenever a diagnosis of prolactinoma is considered. On the other hand, surgery is the preferred treatment for nonfunctioning tumours. The clinical presentation is another issue to be taken into account. These considerations were discussed with the patient. Since the tumour was found after a probably prolonged presence of her visual field defect, reversion of the optic chiasm affection by an urgent decompression surgery was not expected and was not counselled.
A large pituitary tumour in a young woman with hyperprolactinaemia was more probably compatible with the presence of a prolactinoma as the first diagnostic possibility. Hence, a therapeutic trial with the dopamine agonist Bromocriptine was prescribed as a first approach. Since the tumoural size was considerable, Bromocriptine treatment would probably be followed by a debulking surgery procedure as required. The Bromocriptine dose was progressively increased from 1.25 to 3.75 mg orally daily. Bromocriptine dose for a prolactinoma ranges 2.5-15.0 mg per day. Due to the pregnancy associated risk of tumour enlargement, a non-hormonal contraceptive method was firmly recommended.
The symptoms disappeared at the time Bromocriptine treatment was initiated. Serum PRL levels normalized (10.90 ng/mL) after two months of treatment. Perimetry tests remained stable at six month intervals. The hypophyseal lesion showed a maximum diameter of 1.3 cm after six months of treatment. Serum PRL levels persisted within normal range. The tumour volume decreased to 0.9 cm in 18 months (Fig. 2). Bromocriptine treatment was then discontinued by the patient’s decision at the time she was aware of being pregnant.
As pregnancy advanced, the patient remained with neither headache, galactorrhoea nor did she present apparent visual field deterioration. Increments of PRL levels were in line with the physiological pregnancy-induced elevations. Serum PRL levels ascended to 126.3 ng/mL at the 12th week of gestation. During the 2nd and 3rd trimesters, serum PRL concentrations were around 100 ng/mL. The patient’s temporal hemianopsia seemed to be stable in a perimetry test performed during the 2nd trimester of pregnancy. However, the previous visual field test had been performed six months and not immediately before pregnancy. Because of doubts about pregnancy-induced reappearance or progression of the visual defect, a magnetic resonance image was obtained at the 32nd week of gestation. The radiologist observed a herniation of the optic chiasm into the sella. This herniation, along with the prolonged compression by the tumour, may have been the etiology for the persistence of the visual field defect. Compared to the immediately previous to pregnancy image (Fig. 2), the adenoma size and disposition had not changed during gestation. Thus, Bromocriptine treatment was not resumed.
A healthy baby girl was born at term. The mother opted for breastfeeding despite the associated risk of tumoural growth stimulation. Serum PRL levels did not exceed the range being expected during breastfeeding, with a maximum level of 107.8 ng/mL. The normality of additional pituitary function tests was verified.
The evolution of the symptoms, perimetry test and image was closely followed. Once breastfeeding was concluded, PRL returned to the strict normal values that had been seen before pregnancy and despite the presence of a residual adenoma. A new pregnancy was diagnosed after breastfeeding. No hyperprolactinaemia or tumour enlargement was detected. The patient underwent voluntary abortion due to personal reasons at the 16th week of gestation. Treatment with Bromocriptine or alternative dopamine agonists was not resumed long after delivery. To the date, additional 72 months of follow-up confirm this stability of the disease.
In retrospect, the findings suggest the diagnosis of a nonfunctioning adenoma with a favourable and sustained response to Bromocriptine.
Discussion
An unusual case of Bromocriptine inducing a significant tumour response in what is hypothesized to a nonfunctioning pituitary adenoma is described. Initially, prolactinoma seemed to be the first possibility to be considered, although PRL values were lower than expected for a giant prolactinoma. The final diagnosis is mainly based on the absence of tumoural enlargement neither after an early Bromocriptine treatment withdrawal nor during pregnancy. Such favourable evolution would be atypical in the case of a macroprolactinoma.
The mild ascend of PRL values after Bromocriptine treatment withdrawal, and particularly within pregnancy, further support the diagnosis of a nonfunctioning adenoma. The moderately high serum PRL levels that were found before treatment could be attributed to disconnection of the nonfunctioning adenoma from the hypophyseal stalk (Karavitaki, et al., 2006). The latter causes a disruption of the dopaminergic effect that inhibits PRL release under normal circumstances. These mild PRL levels were not aligned with the large tumoural size at presentation if a prolactinoma was considered. Indeed, macroprolactinomas are usually associated with serum PRL values higher than 250 that could even exceed 1,000 ng/mL (Ivan, 2005). Another issue was that the patient became pregnant. A 10-fold increase in serum PRL levels can be observed throughout normal gestation (Molitch, 2003; Foyouzi, Frisbaek and Norwitz, 2004). Probably further, unpredictable, increments could be seen in the case of a macroprolactinoma (Pituitary Society, 2006). Serum PRL levels did not ascend to those values in this case.
The coexistence of a macroadenoma and mild hyperprolactinaemia leads to a differential diagnosis between nonfunctioning adenoma and prolactinoma with presence of a “hook effect”. The latter is characteristic of giant prolactinomas. Orts Costa, Camara Gomez, Laiz Marro and Ceron Perez (2001) defined the “hook effect” as a phenomenon in which high serum PRL level exceeds an immunoassay’s capacity for accurate detection producing low spurious results. In this case, this phenomenon was ruled out by performing serial PRL measurements in four-fold diluted serum. Besides, normoprolactinaemia after withdrawal of Bromocriptine treatment made the “hook” hypothesis unlikely.
In the reported case, the disease evolution was suggestive of nonfunctioning pituitary macroadenoma. Although infrequent, it is worth mentioning that a silent corticotroph adenoma can also stimulate PRL release mediated by proopiomelanocortin-derivatives. This alternative diagnosis could not be ruled out because tumoural cells immunohistochemical analyses were not available.
The evolution of the tumour within pregnancy was crucial to guide the suggested diagnosis. The interruption of dopamine agonists is commonly associated with a recurrence of the hyperprolactinaemia and with tumour enlargement in the case of a macroprolactinoma, as it was documented by Johnston, et al. (1984), Bevan, Webster, Burke and Scanlon (1992), Ivan, et al. (2005) and by Pituitary Society in issued guidelines (2006). Besides, the natural history of large prolactinomas in pregnant women includes a tumoural enlargement in many cases because pregnancy is the main stimulus for proliferation and growing of hypophyseal lactotroph cells. New appearance of visual symptoms is described in 15-30% of the pregnant women (Bronstein, Salgado and de Castro Musolino, 2002; Molitch, 2003). This complication may need surgery (Molitch, 2003). However, surgery in a pregnant woman is associated with a higher risk of maternal death and spontaneous abortion (Bronstein, Salgado and de Castro Musolino, 2002; Foyouzi, Frisbaek and Norwitz, 2004). Furthermore, surgery does not necessarily restore the visual dysfunction and is not curative in more than one third of the tumours (Foyouzi, Frisbaek and Norwitz, 2004). It is clear that surgery is needed in patients that have a rapid (within days) instauration and detection of a visual field defect. Otherwise, we suggest a therapeutic trial or dosing adjustment of a dopamine agonist drug as the first option whenever the size of a prolactinoma increases within pregnancy.
Bromocriptine causes a reduction in PRL synthesis. The drug is also successful in substantially reducing the size of a macroprolactinoma in more than a half of treated tumours (Molitch, et al., 1985). This effect is mediated by an induction of perivascular fibrosis, partial necrosis and apoptosis of tumoural cells. Apparently, a response to dopamine agonists is also possible in 8-20% of nonfunctioning adenomas (Bevan, Webster, Burke and Scanlon, 1992; Masding, Lees, Gawne-Cain and Sandeman, 2003). Of interest, 70% of the latter express D2 dopamine subtype receptors (Ivan, et al., 2005). In vitro, the antitumoural effect of Bromocriptine has been proven on nonfunctioning pituitary adenoma cells. A more intense response of cultured cells was obtained with Bromocriptine when compared with somatostatin analogs by Gruszka, et al. (2006). In a series of operated nonfunctioning tumours studied by Greenman (2005; 2007), a reduction or stabilization of remnant size was obtained in 78% of the Bromocriptine treated vs. 38.4% of the non treated group. In this case, the pituitary macroadenoma maximum diameter showed a 60% reduction after 18 months of treatment. However, the evidence available and the clinical expertise with dopamine agonists in nonfunctioning adenomas are still scarce.
In contrast with macroprolactinomas, the absence of growth during pregnancy is a characteristic of nonfunctioning adenomas (Molitch, 2003). But lactotroph cells still grow. Thus, it is possible that a visual impairment develops due to the optic chiasm being simultaneously displaced by the lactotroph hyperplasic cells and the nonfunctioning adenoma. Indeed, it has been described that Bromocriptine may liberate this optic chiasm compression by a nonfunctioning adenoma in a pregnant woman (D’Emden and Harrison, 1986; Masding, Lees, Gawne-Cain and Sandeman, 2003). Molitch (2003) explains this visual chiasm decompression by proposing that Bromocriptine leads to a rapid shrinkage of the lactotroph hyperplasic cells. Neither progression of the initial visual defect nor other evidence of tumoural re-growth was observed in our patient. Consequently, Bromocriptine resumption and/or decompression surgery were not required.
An advantage of Bromocriptine is its safety profile at the dosages required for the treatment of hypophyseal adenomas. The most common side effects include nausea and arterial hypotension. These effects may be diminished by escalating the dosage. The drug may induce sleep. Thus, it is preferably administered at night. Safety of Bromocriptine is also accepted during pregnancy (Pituitary Society, 2006). In addition, Bromocriptine maintenance for at least 12 months before conception may protect her against tumoural re-growth of a prolactinoma during gestation (Ivan, et al., 2005; Bronstein, Salgado and de Castro Musolino, 2002). We cannot totally exclude this situation of a hypothetical prolactinoma in our patient. However, the protective effect of Bromocriptine treatment has been described in women with a prior-to-pregnancy remission of the tumour. The image of a remaining adenoma before pregnancy made this possibility unlikely in this case.
Conclusion
To summarize, we present a pituitary macroadenoma with extrasellar invasion and moderate hyperprolactinaemia. The clinical course suggested the final diagnosis of a nonfunctioning adenoma with an unusual magnitude of the response to pharmacologic treatment with Bromocriptine. The residual adenoma remained stable once Bromocriptine treatment had been discontinued, also during pregnancy and at six year follow-up. Consequently, we consider that a therapeutic trial with Bromocriptine may be effective for pituitary nonfunctioning macroadenomas, especially when the risk-benefit balance of surgery is questioned. Particularly, Bromocriptine treatment may be adequate for non-defined and nonfunctioning macroadenomas diagnosed in fertile and pregnant women.
References
1. Bevan J.S., Webster J., Burke C.W. and Scanlon M.F. (1992) “Dopamine agonists and pituitary tumor shrinkage,” Endocrine Reviews, 13 (2) 220-240.
Publisher – Google Scholar
2. Bronstein M.D., Salgado L.R. and de Castro Musolino N.R. (2002) “Medical management of pituitary adenomas: the special case of management of the pregnant woman,” Pituitary, 5 (2) 99-107.
Publisher – Google Scholar
3. D’Emden M.C. and Harrison L.C. (1986) “Rapid improvement in visual field defects following bromocriptine treatment of patients with non-functioning pituitary adenomas,” Clinical Endocrinology (Oxford), 25 (6) 697-702.
Publisher – Google Scholar
4. Foyouzi N., Frisbaek Y. and Norwitz E.R. (2004) “Pituitary gland and pregnancy” Obstetrics & Gynecology Clinics of North America, 31 (4) 873-892, xi.
Publisher – Google Scholar
5. Greenman Y., et al. (2005) “Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth,” Clinical Endocrinology (Oxford), 63 (1) 39-44.
Publisher – Google Scholar
6. Greenman Y. (2007) “Dopaminergic treatment of nonfunctioning pituitary adenomas,” Nature Clinical Practice Endocrinology & Metabolism, 3 (8) 554-555.
Publisher – Google Scholar
7. Gruszka A., et al. (2006) “The effect of selective sst1, sst2, sst5 somatostatin receptors agonists, a somatostatin/dopamine (SST/DA) chimera and bromocriptine on the “clinically non-functioning” pituitary adenomas in vitro,” Life Sciences, 78 (7) 689-693.
Publisher – Google Scholar
8. Ivan G., et al. (2005) “Treatment of pituitary tumors: dopamine agonists,” Endocrine, 28 (1) 101-110.
Publisher – Google Scholar
9. Johnston D.G., et al. (1984) “Effects of dopamine agonist withdrawal after long-term therapy in prolactinomas,” The Lancet, 2 187-192.
Publisher – Google Scholar
10. Karavitaki N., et al. (2006) “Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma,” Clinical Endocrinology (Oxford), 65 (4) 524-529.
Publisher – Google Scholar
11. Masding M.G., Lees P.D., Gawne-Cain M.L. and Sandeman D.D. (2003) “Visual field compression by a non-secreting pituitary tumour during pregnancy” Journal of the Royal Society of Medicine, 96 (1) 27-28.
Publisher – Google Scholar
12. Molitch M.E., et al. (1985) “Bromocriptine as primary therapy for prolactin secreting macroadenomas: results of a prospective multicenter study,” The Journal of Clinical Endocrinology & Metabolism, 60 698-705.
Publisher – Google Scholar
13. Molitch M.E. (2003) “Pituitary tumors and pregnancy,” Growth Hormone & IGF-1 Research, 13 Suppl A S38-44.
14. Orts Costa J.A., Camara Gomez R., Laiz Marro B. and Ceron Perez J.A. (2001) “Hyperprolactinemia coexisting with hypophyseal adenoma or microadenoma” Medicina Clinica (Barcelona), 117 (5) 196.
15. Pituitary Society (2006) “Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas,” Clinical Endocrinology (Oxford), 65 (2) 265-273.
Google Scholar