Introduction
Wilson’s disease (WD) or hepatolenticular degeneration is an inherited autosomal recessive disease characterized by a defect in the biliary copper excretion, leading to copper accumulation in many organs. The gene responsible for WD encodes a copper-transporting ATPase (ATP7B) and was identified on chromosome 13q14.3. ATP7B protein resides mainly in hepatocytes and is involved in the transmembrane transport of copper within hepatocytes. The defective ATP7B function causes a decreased hepatocellular excretion of copper into bile and eventually an excessive copper deposition mainly in the liver and the brain, leading to the hepatic and neurological features of WD. An additional phenomenon is an altered incorporation of copper into the copper-transporting glycoprotein ceruloplasmin that may lead to an abnormally low plasma concentration of ceruloplasmin. However, this ceruloplasmin deficiency is not always present and a small percentage of WD patients present with normal levels of ceruloplasmin. The prevalence of WD is approximately 1 in 30,000-100,000 individuals and the frequency of carriers of the ATP7B gene mutation is approximately 1 in 90-150 [European Association for Study of Liver, 2012].
On the other hand, sarcoidosis is an idiopathic granulomatous disease affecting multiple organ systems. The disease presents with neurologic manifestations (neurosarcoidosis) in only 5% of patients, 97% of whom also have extraneural disease. The organs most often affected are the lungs (90%), followed by the lymph nodes, skin, eyes, upper respiratory tract, bones, joints, bone marrow, spleen and liver. The prevalence of sarcoidosis varies between 15-20/100.000 in northern Europeans and 1-5/100,000 in southern Europeans [Rybicki BA, Iannuzzi MC, 2007].
We report the case of a patient who presented with tremor and was diagnosed with both Wilson’s disease and sarcoidosis. To our knowledge, this is the third reported case of coexistence of Wilson’s disease and sarcoidosis.
Case Report
A 34 year old Caucasian female was admitted to our department because of rest and intention tremor for the last 1.5 years. The tremor initially involved the right arm and then affected also the left arm and the head. The tremor gradually worsened, compromising skilled activities such as writing. Tremor was exacerbated by physical and psychological stress and improved with mild alcohol consumption. The patient had been previously treated with propranolol 30 mg tid under the diagnosis of “essential tremor” without any clinical improvement. Her family history was unremarkable and there was no consanguinity between the patient’s parents. Her past medical history included hypothyroidism, surgical removal of an endometrial polyp, menstrual irregularities since adolescence and iron deficiency anemia. The patient was a high-school graduate and had worked as a private employee. A detailed occupational and environmental history didn’t reveal any extrinsic factors, such as exposure to various metals , that could be associated with the presenting symptoms. She also reported mild anxiety and depression.
The neurological examination revealed rest, position and task-dependent tremor in the distal upper extremities and a subtle head tremor with rotating features. Mild bradykinesia of the upper limbs, particularly of the left arm, was also noticed. Furthermore, bilateral cogwheel rigidity after activation and a dystonic posture of the left arm (abduction of the little finger) were present. There were no dysautonomic symptoms. The deep tendon reflexes were symmetrical and there were no pyramidal signs or abnormal pyramidal reflexes. The eye movement examination showed mild slowing of horizontal saccades. The glabellar tap sign was negative. Romberg test was negative and the pull test positive (one step backwards). Sensory testing did not reveal any deficits and the muscle strength was normal. A Mini Mental State Examination and an Alzheimer’s disease Assessment Scale-cognitive subscale test (MMSE/ADAScog) were performed and revealed normal cognitive function. Although the patient mentioned occasional symptoms of anxiety and depression, there was no official evaluation on this aspect since her symptoms were considered very mild.
The complete blood count showed microcytic hypochromic anemia and the white blood cells were at the lower limits of the normal range. Routine blood chemistry including liver function tests was normal (ALT 15 U/l, AST 21 U/l , gamma-GT 26 U/l, ALP 55 U/l bilirubin 0,68 mg/dl, Prothrombin time 13, INR 1.1 ). The patient was euthyroid and serum prolactin levels were also within the normal range.
Serum ceruloplasmin levels were low (15.5 mg/dl, reference range 22-58) and serum copper levels were also low (0.6 mcg/ml, reference range 0.7-1.4). Copper levels in a 24h urine collection were increased (400 μg/24h, reference range < 100 μg/24h). Because these findings were suggestive of Wilson’s disease, slit lamp examination was performed and revealed Kayser-Fleischer rings in both eyes with normal visual acuity and normal findings in fundoscopy.
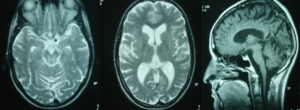
A subsequent magnetic resonance imaging of the brain revealed, on T2 weighted images, small dispersed hyperintense lesions in the cerebral hemispheres (especially in the basal ganglia) and the midbrain around the cerebral aqueduct. Other findings included atrophy of the brain parenchyma with enlargement of the subarachnoid spaces and the ventricular system, and atrophy of the vermis and the cerebellar hemispheres (Figure 1).
Since the initial differential diagnosis included several entities such as early onset Parkinson’s disease, Parkinsonian and Parkinson-plus syndromes, spinocerebellar ataxias, dystonic syndromes and peripheral neuropathy, the following tests were also performed and findings were unremarkable: serum follicle-stimulating and luteinizing hormone levels, serum vitamin B12 and folic acid levels, blood and 24hour urine test for heavy metals (Pb, Cd, Ars, Hg), blood Mg and Zn levels, antineuronal antibodies (anti-Yo, anti-Ri, anti-Hu), DAT scan to rule out other causes of tremor such as young onset Parkinson’s disease or primary dystonia presenting mainly with tremor, and neurophysiological testing (electromyogram and nerve conduction velocity) for signs of peripheral neuropathy associated either to the tremor or the newly diagnosed sarcoidosis.
Based on the above findings, diagnosis of Wilson’s disease and pulmonary sarcoidosis was established. The patient began treatment with trientine for Wilson’s disease. Initially the patient was started on a low dose of 300mg/day for one week that was increased by 300 mg each week until the final dose of 900 mg/day divided in three doses. Meanwhile, the response to treatment was monitored by measuring 24-hour urinary copper excretion and serum copper levels every week. At follow up almost 3 and 6 months after diagnosis, the urinary copper levels were 340 μg/24h and 223 μg/24h respectively (reference range < 100 μg/24h) and the neurological symptoms improved significantly. No specific treatment was given for sarcoidosis. The patient consented to undergo liver biopsy almost one year post treatment that demonstrated significant fibrosis, non-specific chronic inflammation and glycogenated nuclei in hepatocytes. There was only minor focal hepatocellular necrosis and minor steatosis. The rhodanine, PAS-diastase and hemosiderin stains didn’t demonstrate pathological depositions. These findings were suggestive of Wilson’s disease under treatment but not diagnostic. The patient receives a maintenance dose of trientine 900 mg per day and the 24h-urinary copper excretion ranges between 150-300 μg/24h on follow-up studies, while the serum copper levels are between 0,4 — 0,7 μg/ml (reference range 0.7-1.4).
Discussion
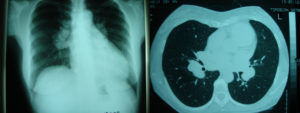
The presence of low ceruloplasmin levels, Kayser-Fleischer ring, high 24-hour urine copper excretion, the brain MRI and the liver biopsy findings strongly support the diagnosis of Wilson’s disease in our patient [European Association for Study of Liver, 2012] , [Roberts EA, Schilsky ML, 2008]. Genotype testing for Wilson’s disease was not performed. At the same time, the findings from the chest X-ray and CT scan, the bronchoalveolar lavage and the biopsy of mediastinal lymph nodes as well as the increased serum ACE levels and the elevated 24h urine calcium excretion were all suggestive of pulmonary sarcoidosis. Therefore, our patient appears to have both Wilson’s disease and pulmonary sarcoidosis.
To our knowledge, this is the third case of coexistence of Wilson’s disease and sarcoidosis that has been reported [Danks DM et al., 1990], [Giouleme O. et al., 2009]. The first report by Danks et al. in 1990, described a forty-three year-old male who presented with symptoms and signs of cirrhosis and no neurologic symptoms and was finally diagnosed with Wilson’s disease. He had a history of pulmonary sarcoidosis which had been diagnosed nine years earlier and treated with corticosteroids. The second patient, described by Giouleme et al. in 2009, suffered from sarcoidosis-induced pericarditis and portopulmonary hypertension. It was thought that portopulmonary hypertension was a vascular consequence of the advanced liver disease caused by Wilson’s disease. The authors’ further investigation of the acute pericarditis provided evidence of sarcoidosis, as another factor of secondary pulmonary arterial hypertension. This was indicated by patient’s rapid improvement of pericarditis after corticosteroid treatment, at one-month follow-up visit, and by gradual decrease of mean pulmonary arterial pressure, at three-month follow-up visit. Reported patient had symptoms of sarcoidosis as well as the symptoms of Wilson’s disease.
The frequency of the overlapping cases is expected to be very low, given the prevalence of sarcoidosis and the prevalence of Wilson’s disease.
Furthermore, these two rare entities don’t seem to share common pathways and their association appears incidental. It is interesting though that elevated serum copper levels can be found in various cancers, immune system diseases and lymphoproliferative disorders. Donghi et al. in 1995 reported an increase of serum copper concentration in Löfgren syndrome (a type of acute sarcoidosis with bilateral hilar lymphadenopathy and erythema nodosum). The authors concluded that, although the finding of an increase of serum copper in patients with mediastinal adenopathies is usually indicative of Hodgkin’s disease, it can be related also to other conditions such as sarcoidosis. [Donghi et al., 1995]. On the other hand, environmental and occupational exposure to aerosolized copper and other metals (aluminum, barium, beryllium, cobalt, copper, gold, lanthanides, titanium, and zirconium) has been strongly correlated with granulomatous lung diseases that mimic sarcoidosis. [Newman LS. 1998, Metals that cause sarcoidosis]. Moreover, several cases of systemic sarcoidosis have been related with cutaneous foreign-body reactions to numerous elements, including copper, calcium, phosphorus, silicon, and aluminium. [Masel G., 2005 Australasian Journal of Dermatology].
Conclusion
In the case discussed here, the coexistence of Wilson’s disease with a seemingly idiopathic disorder such as sarcoidosis raises the question whether there is a potential link between these two diseases. One possible assumption is that the excessive copper due to Wilson’s disease could have acted as an intrinsic factor that triggered an immunologic response in an immunologically predisposed patient and led to the manifestation of sarcoidosis. In any case, the coexistence of both diseases raises special considerations regarding the follow-up and future monitoring of the patient since they could share common hepatic or neurological manifestations.
References
1. Danks, DM, Metz, G, Sewell R. and Prewett EJ. (1990) “Wilson’s disease in adults with cirrhosis but no neurological abnormalities,” British Medical Journal (International Edition), 301(6747):331-2.
Punblisher – Google Scholar
2. Donghi, M, Giura, R. and Antonelli P. (1995) “Increase of serum copper concentration in Löfgren syndrome,” Sarcoidosis, 12(2):147-9.
3. European Association for Study of Liver. EASL. (2012) “Clinical practice guidelines: Wilson’s disease,” Journal of Hepatology, 56:671-85.
4. Giouleme, O, Anagnostis, P, Patsiaoura, K, Vasiliadis, T, Grammatikos, N, Kakavas, N, Mpoumponaris, A, Eugenidis, N. and Basayannis E. (2009) “Sarcoidosis induced pericarditis in a patient with portopulmonary hypertension: a case report,” Cases Journal, 2:8640.
Publisher – Google Scholar
5. Masel, G. (2004) “Copper-induced cutaneous sarcoidosis,” Australasian Journal of Dermatology, 46:38—41.
6. Newman, LS. (1998) “Metals that cause sarcoidosis,” Seminars in Respiratory Infections, 13(3):212-20.
7. Roberts, EA. and Schilsky ML; American Association for Study of Liver Diseases (AASLD). (2008), “Diagnosis and treatment of Wilson’s disease: An update,” Hepatology, 47 (6): 2089-111.
Publisher – Google Scholar
8. Rybicki, BA. and Iannuzzi MC. (2007) “Epidemiology of sarcoidosis: Recent advances and future prospects,”Seminars in Respiratory and Critical Care Medicine, 28 (1):22-35.
Publisher – Google Scholar