Introduction
Neuroblastoma is the most common extracranial solid tumor of children deriving from neural crest tissues. Its prognosis is poor especially in older children with advanced disease who receive intensive combined chemotherapies (Maris et al.). Erythrocyte agglutination indicates the clumping of red blood cells due to cold agglutinins, in particular IgM antibodies. Erythrocyte agglutination is an uncommon state in solid tumors. We present here a 4-year-old-boy who was diagnosed with neuroblastoma along with erythrocyte agglutination on blood smear during his second chemotherapy cycle.
Case Report
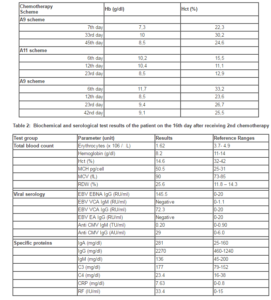
A 4-year-old-boy was admitted to the Children Hospital of Hacettepe University in August 2009 with the complaints of fever and anorexia for one month. His past medical history was uneventful. On examination, the physical signs were not remarkable except for a left sided abdominal mass of 10×10 cm. Laboratory findings included: hemoglobin 8.1 g/dL, mean corpuscular volume (MCV) levels 70.9 fL, white blood cell count 11.4 × 109/L, red blood cell count 3.37× 106/L and platelet count 611 × 109/L. Serum hepatic and renal functions were normal, but serum lactate dehydrogenase was found to be high (24170 IU/L). Urinary vanillylmandelic acid and homovalinic acid levels (14,6 mg/g creatinine and 46,3 mg/g creatinine) and erythrocyte sedimentation rate (100 mm/hr) were also increased. Abdominal computed tomography (CT) showed a 12x9x8 cm, heterogeneous mass originating from the left adrenal gland. Neuroblastoma was diagnosed by an ultrasonography-guided needle biopsy with unfavorable histology. His chest x-ray, bone scans and bone marrow biopsy were all normal. An elevated MYC-n amplification was observed on biopsy specimen. He was started on the high-risk neuroblastoma chemotherapy protocol of the Turkish Pediatric Oncology Group. As the first chemotherapy cycle, he received A9 scheme containing vincristine (1.5 mg/m2), ifosfamide (1.5 g/m2), adriamycin (30 mg/m2) and dacarbazine (200 mg/m2). On the sixth day of the second chemotherapy cycle, as A11 scheme containing cyclophosphamide (300 mg/m2), etoposide (80 mg/m2) and cisplatin (30 mg/m2), a discrepancy between hemoglobin (Hb) and hematocrit (Hct) values was detected associated with increased MCH (67 pg) and MCV (101,6 fL) values, and decreased erythrocytes (1,52 x 106/μL) (Table 1). Blood smear analysis showed normocytic normochromic erythrocytes with few spherocytosis and marked erythrocyte agglutination. After warming at 37 0C for an hour, peripheral blood smear was repeated and erythrocyte agglutination disappeared. On the next day, venous sample was drawn from the patient in the laboratory with the consent of parents; then total blood count and blood smear analysis were immediately performed; neither Hb-Hct discrepancy nor erythrocyte agglutination was observed. The patient was thought to have acute cold agglutination. However, there were no symptoms of Mycoplasma infection such as pneumonia symptoms; cough, fever, chills, chest pain. High levels of serum EBV-IgG and CMV-IgG showed previous viral infection (Table 2). Cold agglutination test was positive, whereas direct Coombs test was negative. Serum IgG and IgA values were high, while serum IgM values were normal (Table 2). The problem did not appear again and the patient received a total of 6 courses of intensive alternating A9 and A11 chemotherapy regimens. The patient received intensive systemic chemotherapy and abdominal radiotherapy; but complete remission could not be achieved.
Discussion
In this case, the coexistence of low hematocrit levels with high MCH and MCV values suggested auto-agglutination of erythrocytes. Low hematocrit levels can be associated with hemolytic anemia, infants without adequate iron, children during rapid growth, women in childbearing and patients with chronic kidney disease, in which erythropoietin levels are low/lack. Elevated WBC counts (>50.000 /ïL) can cause high MCV and MCH values. Defects in nuclear maturation, such as in megaloblastic anemia, result in large erythrocytes with high MCV and MCH and normal hemoglobin levels. Our prediction was confirmed by blood smear analysis containing large erythrocyte agglutinates. Erythrocyte agglutination was first observed on the sixth day of the second cycle of chemotherapy (A11) and then disappeared.
Erythrocyte agglutination is commonly due to cold agglutinins that attach erythrocyte below 37 0C. Complement fixation also occurs at that temperature; those complement coated cells removed by macrophages in liver or spleen, or by intravascular hemolysis (Packman CH). Cold agglutinins are generally associated with lymphoproliferative diseases such as chronic lymphocytic leukemia, non-Hodgkin lymphoma or systemic lupus erythematosus, or the infections due to Mycoplasma pneumonia, cytomegalovirus, infectious mononucleosis or mumps (Roelke et al.). In this case, no evidence due to these reasons was found. Erythrocyte agglutination only appeared following the second chemotherapy course containing cyclophosphamide, etoposide and cisplatin, which could be triggered by these chemotherapeutics. Cold agglutinin test showed that the cold antibodies recognized the antigen, which was fully expressed in adult erythrocytes, but Coombs test for IgG and C3 was found to be negative. Cold agglutinins are usually IgM, which recognizes erythrocyte antigens within the li system or the Pr complex (Roelke et.al). Since IgM did not increase, it was thought that IgG class might have been responsible for erythrocyte agglutination. The studies of Ambrus and Crisp that demonstrated the increase of IgG type anti-I cold antibodies in patients with lymphocytic malignancies support our result (Ambrus et.al. and Crisp et.al.).
For our case, we could not document the cause of erythrocyte agglutination on separate occasions. Detection of erythrocyte agglutination following the second chemotherapy cycle and the disappearance without any intervention might suggest that those chemotherapeutics induced the antibody-mediated process during that period. However, we observed no similar blood count results after the following chemotherapy courses.
We could not find relevant reports in the literature concerning the association of chemotherapeutic drugs with erythrocyte agglutination. As an example of drug effects, Nishiyama et al reported the occurrence of erythrocyte agglutination on peripheral blood smear, and an abnormal erythrocyte distribution histogram that suggested the influence of cefpirome sulfate (Nishiyama et.al.).
To our knowledge, this is the first report in the sparsely literature in which erythrocyte agglutination due to cold agglutinins was detected in a child diagnosed with neuroblastoma. This appeared as decreased red blood cell count and falsely elevated MCV on the automatic cell counter. We would like to emphasize that this is a rare observation.
References
1. Ambrus M and Bjtai G. (1969) “A case of an IgG-type cold agglutinin disease” Haematologica, 3 225—228.
2. Crisp D and Pruzanski W. (1982) “B-cell neoplasms with homogeneous cold-reacting antibodies (cold agglutinins)”.Am J Med, 72 915—919.
Publisher – Google Scholar
3. Maris JM, Hogarty MD, Bagatell R, Cohn SL. (2007) “Neuroblastoma” Lancet, 369 (9579) 2106-20.
4. Nishiyama M, Koh T, Kabutomori O, Yanagihara T. (1997) “A case of red blood cell (RBC) agglutination on peripheral blood smear and effect of cefpirom sulfate”. Rinsho Byori 45(5) 498-500ff.
Google Scholar
5. Packman CH. (2005) “Immune Haemolytic Anaemia” Encyclopedia Of Life Sciences: 1 (1-9).
Google Scholar
6. Roelke D. (1989) “Cold agglutination” Transfuse Med Rev, 2 140-166.
Google Scholar