Introduction
Neuroendocrine tumours arise from enterochromaffin cells of the gastrointestinal tract and other organs. Somatostatin-producing tumours are relatively rare neuroendocrine tumours, with an estimated annual incidence of 1 in 40 million. After the first description of two pancreatic somatostatinomas in 1977, fewer than 200 cases have been reported. The clinical presentation includes nonspecific symptoms such as abdominal pain, nausea, diarrhea and weight loss. Typical somatostatin syndrome, first described in 1979, consists of diabetes, cholelithiasis and steatorrhea and is caused by excess somatostatin released from the tumour. However, this classic triad is present in only a small proportion of patients.
Somatostatinomas arise more frequently in the pancreas, followed by the duodenum; the ampulla of vater and the jejunum are infrequent locations6. Extrapancreatic somatostatinomas are malign tumours, but they are generally smaller and less often associated with metastases than pancreatic somatostatinomas. However, only limited information about the long-term prognosis of patients with advanced somatostatinoma has been reported.
Case Report
We are presenting a case of a male patient diagnosed with somatostatinoma when he was 30 years old. The patient was admitted to Badajoz University Hospital (Spain) because of repeated episodes of abdominal pain, nausea, vomiting and diarrhea with steatorrhea, preceded by sweating and flushing, during the previous year.
The laboratory tests revealed a blood glucose level of 128 mg/dl as well as abnormal liver enzymes. Multiple metastatic masses appeared on the ultrasound. Abdominal computed tomography confirmed the liver enlargement due to innumerable liver metastases (Figure 1). A liver biopsy showed a low-grade neuroendocrine tumour with a proliferation index Ki-67 lower than 1%. Immunohistochemical staining demonstrated a diffuse positivity for neuron-specific enolase, chromogranin A, synaptophysin and somatostatin (Figure 2). The serum somatostatin level was 724 pg/ml (normal range: 17-80 pg/ml).Serum gastrine level was in the normal range.
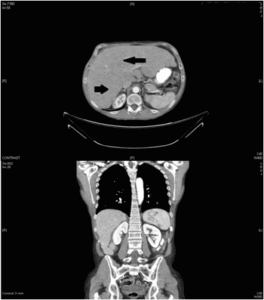
Figure 1. Abdominal Computed Tomography Showing Liver Enlargement and Multiple Focal Liver Metastases. Black Arrows Show the Location of Liver Metastasis
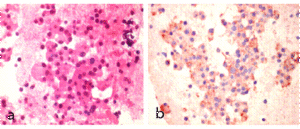
Figure 2. Histological Appearance of Liver Biopsy (Magnification X 60). (A) Standard Haematoxylin and Eosin Stain Showing Tumour Cells with Wide Eosinophilic Granular Cytoplasms and Round Eccentric Nucleus. (B) Immunohistochemistry for Somatostatin Showing Tumour Cells Expressing Somatostatin in Granular Cytoplasmatic Distribution
The patient was operated and a 1.5 cm tumour located in the second portion of the jejunum was removed. The pathological examination showed a grade 1 neuroendocrine tumour. Liver metastases were confirmed and infiltration in four lymph nodes was found (T4N1M1). Immunohistochemistry showed strong staining with somatostatin and weak staining with gastrin. Although the somatostatin receptor scintigraphy failed to show any somatostatin receptor tissue (Figure 3), after surgery the patient was treated with the long-acting release formulation of octreotide, a somatostatin analogue. The somatostatin receptor scintigraphy was repeated several times during follow-up, showing similar results.
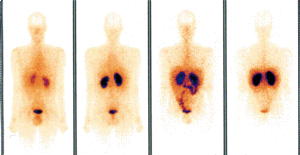
Figure 3. Somatostatin Receptor Scintigraphy Failing to Show Any Somatostatin Receptor Tissue
The patient survived for 12 years, with stable metastatic hepatic lesions and he enjoyed a good quality of life. Serum somatostatin levels increased after 6 years of follow-up, probably reflecting tumoral progression, although they were not checked again afterwards (Figure 4).
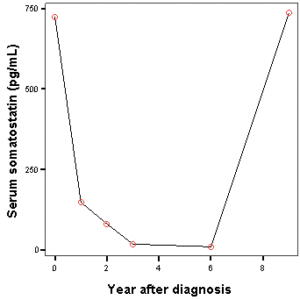
Figure 4. Serum Somatostatin Levels during Follow-up (Normal Range: 17-80 Pg/Ml)
Nevertheless, the patient experienced, from diagnosis, twice a month episodes of abdominal pain, diarrhea with steatorrhea and vomiting. No symptoms of cholelithiasis were shown. During these episodes, blood glucose rose to 400 mg/dl and, without administration of any insulin at that moment, it rapidly decreased to severe hypoglycaemia (< 30 mg/dl). In two of these episodes, the patient suffered from hypoglycaemic coma that recovered with intravenous glucose infusion. Seven years after the diagnosis, the glycosilated hemoglobin was 9.3%, so the patient was treated with insulin glargine (0.13 U/kg).
The tumour was stable on somatostatin analogs for 10 years. Then, the tyrosine-kinase inhibitor sunitinib was prescribed for one year due to symptoms persistence and progression; as the symptoms showed no improvement, sunitinib was later changed to the mTOR inhibitor everolimus. No improvement in the symptoms, biochemmistry levels or radiological images was found in response to sunitinib or everolimus.
Finally, the patient died 12 years after diagnosis because of severe ascitis and dyspnea due to tumour progression.
Discussion
Even in presence of liver metastases at diagnosis, surgical removal of primary somatostatin-producing tumours is recommended, as it may lead to improved survival or quality of life. Somatostatin analogues may reduce tumour secretion and retard tumour growth1.
An overall 5-year survival rate of 40-60% for patients with somatostatinoma has been reported. In the presence of liver metastases, the reported survival rate is 40%. Many patients with duododenal or pancreatic somatostatinomas have advanced disease at diagnosis, but long-term survival rates have been observed after surgical treatment. The size of the tumour appears to be the critical risk factor for the development of metastases, whereas other factors, such as age, gender, location of the tumour and histological characteristics have demonstrated no relation to metastases. The risk of metastases seems to increase significantly with tumours larger than 2 cm. Therefore, the prognosis of patients with advanced somatostatinoma is perhaps not as poor as previously reported, with an average survival period of 1-2 years. The reported long-term survivals, up to 30 years, suggest that duodenal malignant somatostatinomas potentially have a low malignant potential and slow growth. Indeed, primary location in jejunum is very rare and the information related to the long-term prognosis of jejunal somatostatinomas is scarce.
Several causes may lower tumor detectability in somatostatin receptor scintigraphy studies, such as presence of unlabeled somatostatin resulting from production by the tumor itself, different somatostatin receptor subtypes with different affinities for the radioligand and variable tumor differentiation and receptor expression.
Hypoglycemia in somatostatinomas has been reported in very few cases and its mechanism remains unclear. Somatostatin may inhibit the secretion of glucagon and other hormones that can increase the glucose level.
Systemic therapy for somatostatin-producing tumours includes somatostatin analogs, cytotoxic chemotherapy and molecular targeted therapy, such as tyrosine-kinase inhibitors (sunitinib and sorafenib) and mTOR inhibitors (everolimus). In our patient, the tumour was stable on somatostatin analogs for 10 years but showed to response tosunitinib or everolimus.
In summary, we report a case of a low-grade malignancy jejunal somatostatinoma in a 30-year-old man, with liver metastases at diagnosis, treated with surgical resection and somatostatin analogues. The patient presented a long-term survival, with acceptable quality of life but with repeated episodes of gastrointestinal symptoms.
References
Ganda, O. P., Weir, G. C., Soeldner, J. S., Legg, M. A., Chick, W. L., Patel, Y. C., Ebeid, A. M., Gabbay, K. H. & Reichlin, S. (1977). “Somatostatinoma: A Somatostatin-Containing Tumor of the Endocrine Pancreas,” The New England Journal of Medicine, 28; 296(17) 963-7.
Publisher – Google Scholar
Garbrecht, N., Anlauf, M., Schmitt, A., Henopp, T., Sipos, B., Raffel, A., Eisenberger, C. F., Knoefel, W. T., Pavel, M., Fottner, C., Musholt, T. J., Rinke, A., Arnold, R., Berndt, U., Plöckinger, U., Wiedenmann, B., Moch, H., Heitz, P. U., Komminoth, P., Perren, A. & Klöppel, G. (2008). “Somatostatin-Producing Neuroendocrine Tumors of the Duodenum and Pancreas: Incidence, Types, Biological Behavior, Association with Inherited Syndromes, and Functional Activity,”Endocrine-related Cancer, 15(1) 229-41.
Publisher – Google Scholar
House, M. G., Yeo, C. J. & Schlock, R. D. (2002). “Periampullary Pancreatic Somatostatinoma,” Annals of Surgical Oncology, 9(9) 869-74.
Publisher – Google Scholar
Kim, J. A., Choi, W. H., Kim, C. N., Moon, Y. S., Chang, S. H. & Lee, H. R. (2011). “Duodenal Somatostatinoma: A Casereport and Review,” The Korean Journal of Internal Medicine, 26(1) 103-7.
Publisher – Google Scholar
Koc, O., Duzkoylu, Y., Sari, Y. S., Bektas, H., Uzum, G., Tunali, V. & Pasaoglu, E. (2013). “Duodenal Somatostatinoma: A Case Report and Review of the Literature,” Journal of Medical Case Reports, 25;7(1) 115.
Publisher – Google Scholar
Krejs, G. J., Orci, L., Conlon, J. M., Ravazzola, M., Davis, G. R., Raskin, P., Collins, S. M., Mccarthy, D. M., Baetens, D., Rubenstein, A., Aldor, T. A. & Unger, R. H. (1979). “Somatostatinoma Syndrome. Biochemical, Morphologic and Clinical Features,” The New England Journal of Medicine, 9;301(6) 285-92.
Publisher – Google Scholar
Larsson, L.- I., Hirsch, M. A., Holst, J. J., Ingemansson, S., Kühl, C., Jensen, S. L., Lundqvist, G., Rehfeld, J. F. & Schwartz, T. W. (1997). “Pancreatic Somatostatinoma. Clinical Features and Physiological Implications,” The Lancet,26;1(8013) 666-8.
Publisher – Google Scholar
Schillaci, O., Annibale, B., Scopinaro, F., Dellefave, G. & Colella, A. C. (1997). “Somatostatin Receptor Scintigraphy of Malignant Somatostatinoma with Indium-111-Pentetreotide,” Journal of Nuclear Medicine, 38;(6) 886-7.
Publisher – Google Scholar
Tanaka, S., Yamasaki, S., Matsushita, H., Ozawa, Y., Kurosaki, A., Takeuchi, K., Hoshihara, Y., Doi, T., Watanabe, G. & Kawaminami, K. (2000). “Duodenal Somatostatinoma: A Case Report and Review of 31 Cases with Special Reference to the Relationship between Tumor Size and Metastasis,” Pathology International, F50(2) 146-52.
Publisher – Google Scholar
Ulfberg, J., Tot, T. & Wilander, E. (1989). “Thirty-Year Survival with an Untreated Malignant Duodenal Somatostatinoma,”Endocrine Pathology, 9(1) 375-378.
Publisher – Google Scholar






