Introduction
Typical carcinoids are generally associated with a much better prognosis than atypical carcinoids and other types of primary lung cancer (Naalsund et al 2011 and Wurtz et al. 2009). Peripheral pulmonary carcinoids, which account for only a small proportion of bronchopulmonary carcinoids, more frequently exhibit an atypical histology. Thus, typical carcinoid tumors that are peripheral, synchronous, and bilateral are extremely rare (Beshay et al. 2003, Yazici et al. 2010, Sivrikoz et al. 2008, and Camargo et al.2009). Additionally, the optimal extent of resection for a small-sized typical pulmonary carcinoid tumor has not been fully established.
Case Report
A 68-year-old man was admitted for the treatment of bilateral pulmonary nodules that were detected by computed tomography (CT). CT showed an evenly shaped pulmonary nodule, 7 mm in diameter in the right lower lobe, and 2 very small nodules in the left lobes, 3 and 1 mm in diameter. These findings were initially suspected to be inflammatory changes; however, 9 months after the initial examination, the nodule on the right was found to have grown slowly, reaching 11 mm in diameter (Fig. 1A), and a 2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) scan showed a slightly increased activity (SUVmax = 1.5). That right nodule was suspected to be a malignant tumor, and the other nodules were also suspected to be tumor metastases; however, preoperative examinations indicated neither primary lesion nor lymphadenopathy. Wedge resection for the right nodule was performed, and macroscopic examination of it showed a well-circumscribed small nodule (Fig. 1B).
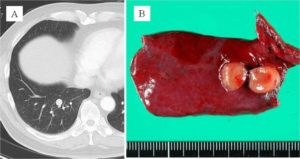
Figure 1: (A) CT Scan Image of Right Small Nodule. (B) Macroscopic Appearance of Resected Specimen and Tumor
Systemic nodal dissection was not performed, because (a) no enlarged lymph nodes were apparent on imaging examinations or during the operation, (b) the tumor was well defined and very small, and (c) the frozen section diagnosis was typical carcinoid. Histopathological examination showed spindle, small ductal and follicle structures and failed to detect any apparent pleural invasion, airway dissemination, or vessel/lymphatic infiltration (Fig. 2).
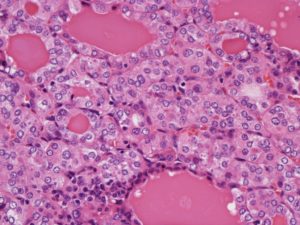
Figure 2: Histopathological Examination of Resected Tumor
Seventeen months later, one of the left lung tumors had grown slightly, reaching 6 mm in diameter (Fig. 3A). Although a FDG-PET scan showed no activity in the tumor, lymph nodes, or any other remote organs, wedge resection for a well-circumscribed small nodule (Fig. 3B) was performed. Histopathological examination revealed findings identical to those of the right lung tumor. In both tumors, no necrosis was detected, and maximally 1 mitotic change was detected per 10 High Power Field. Immunohistochemical analysis of both tumors showed the same findings as positive for CD57, synaptophysin, S100a, and negative for chromogranin A. Taken together, the pathologist at our institution finally diagnosed the tumors as typical carcinoids, although the follicle structures are usually uncommon. Additionally, the characteristic finding of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) was not evident. As neither tumor appeared to have invaded the vasculature or lymphatic ducts, we diagnosed synchronous bilateral typical pulmonary carcinoid tumors. The postoperative course was uneventful, and the patient has been disease-free for 18 months since the last surgery.
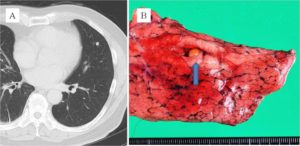
Figure 3: (A) CT Scan Image of Left Small Nodule. (B) Macroscopic Appearance of Resected Specimen and Tumor (Arrow)
Discussion
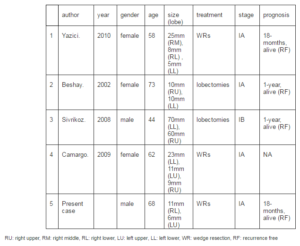
Carcinoids, particularly typical carcinoids, rarely present peripherally; therefore, synchronous, peripheral and typical carcinoid tumors are extremely rare. To the best of our knowledge, only a few cases of synchronous typical carcinoids in both peripheral lungs have been reported (Beshay et al. 2003, Yazici et al. 2010, Sivrikoz et al. 2008, and Camargo et al.2009), and the summary of those reported cases was shown in table 1. Although recent literature has reported the occurrence of multiple carcinoids in a background of DIPNECH (Oba et al. 2013), there was no finding of DIPNECH in the present case. Additionally, a case with multiple peripheral typical carcinoid tumors in one lobe was reported recently (Kim et al. 2013). In this case, multiple endocrine neoplasia (MEN) syndrome is also needed to be considered. It is difficult to exclude the possibility of MEN syndrome in this case completely; however, there was no tumorous disease other than pulmonary carcinoid tumors after whole body examination, including CT (neck, chest, and body), and PET scan.
Table 1: Summarization of Previously Reported Cases of Synchronous, Bilateral, Peripheral Typical Pulmonary Carcinoid Tumors
Carcinoid tumors are not associated with any specific radiologic findings and are often diagnosed as benign lesions because of their slow growth. Recent studies have demonstrated that FDG-PET imaging is useful for evaluating pulmonary carcinoid tumors, particularly nodules greater than 1.5 cm in diameter (Daniels et al. 2007). In this case, only the right tumor showed a slightly increased activity on FDG-PET; therefore, we considered that the right tumor required surgical resection. One of the other small-sized nodules was subsequently resected after gradually increased in size; however, that tumor showed no activity on FDG-PET. Recent literature has demonstrated that the use of PET with somatostatin analogue labeled with gallium-68 can differentiate carcinoid tumors from benign pulmonary lesions (Lococo et al. 2014); however, gallium-68 PET was not performed in this case.
Bronchopulmonary carcinoids generally exhibit less-aggressive behavior, and an overall survival of 92% to 97% for typical carcinoids and 66% to 78% for atypical carcinoids has been reported (Naalsund et al 2011), reflecting the fact that nodal involvement and metastases are rare, especially in the former. Recent studies have shown that, for peripheral typical carcinoids, it is possible to perform conservative resection with preservation of much of the lung parenchyma, and bronchial resection can be performed for more centrally located tumors (Yendamuri et al. 2011). A large case series using a multicenter database demonstrated that, compared with lobectomy, sublobar resection is associated with at least equal survival in patients with typical carcinoids (Fox et al. 2013). Although the actual relationship between lymphatic spread and tumor size in typical carcinoids has not been determined, Wurtz et al. demonstrated that lymphatic spread was significantly greater when the tumor diameter was larger than 3 cm (Wurtz et al. 2009). In this patient, anatomical resection with systemic lymph node dissection was not performed, because the frequency of lymphatic metastasis fromperipheral, small-sized typical carcinoids seems to be very low and no lymphadenopathy was detected on imaging examination or during surgery. However, some investigators have advocated the necessity of lymph node dissection, even for typical carcinoids (Yendamuri et al. 2011), and several reports revealed that lymph node relapse occurred after a long period of remission up to 32 years postoperatively. Although the optimal extent of resection for typical carcinoid tumors has not yet been established, especially for cases of small-sized tumors in the periphery of the lung, we suggest that, at present, careful preoperative evaluation, complete surgical resection, and long-term follow-up should be the preferred treatment strategy for these tumors.
Acknowledgments and Disclosures
The authors thank Dr. Akihisa Saito, and Dr. Kiyomi Taniyama for their assistance with pathological examination. The authors have no conflict of interest.
References
Beshay, M., Roth, T., Stein, R. & Schmid, R. A. (2003). “Synchronous Bilateral Typical Pulmonary Carcinoid Tumors,”European Journal of Cardiothoracic Surgery, 23 (2) 251—253.
Publisher – Google Scholar
Camargo, S. M., Machura, T. N., Moreira, A. L. S., Schio, S. M., Moreira, J. S. & Camargo, J. J. P. (2009). “Multiple Synchronous Bronchial Carcinoid Tumors: Report of a Case,” Thoracic Cardiovascular Surgery 57(1) 58—60.
Publisher – Google Scholar
Daniels, C. E., Lowe, V. J., Aubry, M. C., Allen, M. S. & Jett, J. R. (2007). “The Utility of Fluorodeoxyglucose Positron Emission Tomography in the Evaluation of Carcinoid Tumors Presenting as Pulmonary Nodules,” Chest 131 (1) 255 —260.
Publisher – Google Scholar
Fox, M., Van Berkel, V., Bousamra, M. 2nd., Sloan, S. & Martin, R. C. 2nd. (2013). “Surgical Management of Pulmonary Carcinoid Tumors: Sublobar Resection versus Lobectomy,” American Journal of Surgery, 205 (2) 200—208.
Publisher – Google Scholar
Kim, Y., Choi, Y., Kim, B. J., Oh, I., Somg, S., Nam, J. & Park C. (2013). “Multiple Peripheral Typical Carcinoi Tumors of the Lung: Associated with Sclerosing Hemangiomas,” Diagnostic Pathology 8(1): 97.
Publisher – Google Scholar
Lococo, F. & Treglia, G. (2014). “Which is the Best Strategy for Diagnosing Bronchial Carcinoid Tumours? The Role of Dual Tracer PET/CT Scan,” Hellenic Journal of Nuclear Medicine 17(1) 7-9.
Publisher – Google Scholar
Naalsund, A., Rostad, H., Strom, E. H., Lund, M. B. & Strand, T. E. (2011). “Carcinoid Lung Tumors–Incidence, Treatment and Outcomes: A Population-Based Study,” European Journal of Cardiothoracic Surgery, 39 (4) 565—569.
Publisher – Google Scholar
Oba, H., Nishida, K., Takeuchi, S., Akiyama, H., Muramatsu, K., Kurosumi, M. & Kameya, T. (2013). “Diffuse Idiopathis Pulmonary Neuroendocrine Cell Hyperplasia with a Central and Peripheral Carcinoid and Multiple Tumorlets: A Case Report Emphasizing the Role of Neuropeptide Hormones and Human Gonadotropin-Alpha,” Endocrine Pathology, 24 220-228.
Publisher – Google Scholar
Sivrikoz, M. C., Tulay, C. M., Doner, E. & Ozkan, R. (2008). “Synchronous Bilateral Typical Carcinoid Tumors of the Lung,” The Thoracic and Cardiovascular Surgeon, 56 (1) 62—64.
Publisher – Google Scholar
Wurtz, A., Benhamed, L., Conti, M., Bouchindhomme, B. & Porte, H. (2009). “Results of Systematic Nodal Dissection in Typical and Atypical Carcinoid Tumors of the Lung,” Journal of Thoracic Oncology, 4 (3) 388—394.
Publisher – Google Scholar
Yazici, U., Gulhan, E., Agackiran, Y., Tastepe, I. & Yaran, P. (2010). “Synchronous Bilateral Multiple Typical Pulmonary Carcinoid Tumors,” Annals of Thoracic Surgery 89 (4) 1278—1280.
Publisher – Google Scholar
Yendamuri, S., Gold, D., Jayaprakash, V., Dexter, E., Nwogu C. & Demmy, T. (2011). “Is Sublobar Resection Sufficient for Carcinoid Tumors?,” The Annals of Thoracic Surgery, 92 (5) 1774—1778; Discussion 1778—1779.
Publisher – Google Scholar






