Introduction
The treatment of chronic pain is one of the most common reasons for patients to visit their physician. [1] A mainstay of chronic pain management is the long-term use of opiates. In humans, chronic exposure to opioids has been shown to impair the hypothalamic-pituitary-adrenal (HPA) and the hypothalamic-pituitary-gonadal (HPG) axes. [2] Opioid-induced inhibition of the HPA and the HPG axes is mediated by different opioid mechanisms at the hypothalamic level. It was demonstrated that, administration of fentanyl inhibits HPA axis activity in rhesus monkeys. [3] In our review of the medical literature, there have been only four cases reported regarding opioid-induced secondary adrenal insufficiency (2oAI). In two of these cases, 2oAI was attributed to chronic use of a high dose fentanyl patch. [6, 10] One case of 2oAI was felt to be due to hydromorphone and the other secondary to long term use of tramadol. [7, 9] None of these cases were reported in the United States.
Case Report
A 58-year-old female with a past medical history of non-small cell lung cancer, status post right upper lobe lobectomy and hypertension presented to the Emergency Room (ER) with several episodes of low blood pressure. She also complained of fatigue, weakness and a forty pounds weight loss over the last two years. On physical examination, she appeared cachectic and malnourished with systolic blood pressure in 70’s. The preliminary diagnosis was shock. The etiology for shock remained unclear.
Infection was ruled out and she was afebrile, there was no leukocytosis. The urine and blood cultures were negative for infection, the chest X-ray showed no infiltrates. Troponins were not elevated and the EKG was within normal limits. The echocardiogram showed an ejection fraction (EF) of 60% — 65%, with mild mitral regurgitation and no significant valvular abnormalities. With persistent hypotension and the investigation for other causes of shock ruled out, adrenal insufficiency became a strong diagnostic consideration.
As a result, serum cortisol levels were ordered which resulted as a random cortisol of 1.5 mcg/dl and a repeat 5 AM cortisol of 1.3 mcg/dl. Subsequently, a cosyntropin stimulation test demonstrated a 30 minutes serum cortisol level of 12.1 mcg/dl and 60 minutes serum cortisol level of 16.6 mcg/dl, (18-20mcg/dl) confirming the diagnosis of adrenal insufficiency. A serum sodium of 139 milliequilents/liter (meq/l) and a serum potassium of 4.9 meq/l indicated an intact mineralocorticoid system. To differentiate between primary and secondary adrenal insufficiency, plasma adrenocorticotropic (ACTH) hormone levels for 8am were ordered which were reported to be <5pg/ml, (10-60pg/ml) confirming the secondary adrenal insufficiency.
To evaluate for any pituitary pathology causing the secondary adrenal insufficiency, a MRI of the brain was performed. This showed no evidence of pituitary hemorrhage or any adenoma, although nonspecific white matter hyper-intensities were reported. The differential diagnosis for these hyper intensities were reported to be migraine, microangiopathy, demyelination or inflammatory process. The Patient was on Vicodin (hydrocodone/acetaminophen) 7.5mg/750 mg four times a day and fentanyl patch 75 mcg every 48 hours for many years for her chronic back pain.
We hypothesized that the patient had opioid-induced secondary adrenal insufficiency and she was started on a therapeutic dose of intravenous hydrocortisone. The dramatic improvement of her blood pressure with steroids and fluids along with the supportive laboratory tests, confirmed the clinical suspicion of secondary adrenal insufficiency. Vicodin was discontinued and the fentanyl patch dose was reduced to 25 mcg/hr every 48 hours. Subsequently, the steroid dose was changed to prednisone 5mg orally daily. Non-opiate pain medications were added for the chronic pain. Our team educated the patient concerning the signs and symptoms of adrenal insufficiency. She was advised to take a higher dose of prednisone (20 mg) if she was ill and not feeling well. We also explained the importance of reporting to the ER if she developed nausea and vomiting or if her symptoms were not getting better.
Three months later, the patient was followed up at the outpatient clinic where she denied any episodes of hypotension or any symptoms suggesting hypotension; ironically she was found to be hypertensive and taking lisinopril. At this point, the prednisone was discontinued (and not tapered as she was on a very small dose of 5mg daily) and she was advised to restart it, if she developed any of the signs and symptoms of adrenal insufficiency. The patient was again seen three months later for a follow-up visit. She denied any symptoms suggestive of adrenal insufficiency and she had not taken any prednisone during this time interval. Laboratory investigations revealed a 8am serum cortisol level of 17.7 mcg/dl (18-20mcg/dl) and plasma ACTH level of 37pg/ml (10-60pg/ml). [Figure 1] The patient remains off of prednisone and symptom free as of this writing.
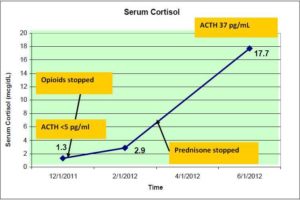
Figure 1. The Variation in the Level of A.M Serum Cortisol and the Level of ACTH in the Patient after Being Taken off of the Hydrocodone and Fentanyl
Discussion
Opioids are commonly prescribed medications for the treatment of chronic pain. Fentanyl, for example, is and potent and effective medication commonly prescribed for pain relief. [4] Constant delivery of the drug in the form of a transdermal patch helps breakthrough pain.
There has been an increase in the prescribing of opiate medications for the treatment of chronic pain. The treating clinician needs to be aware of risks involved with the prescription of long term opiate medication. This includes being aware of the possible effects of long term opiate use on the endocrine system.
The Intrathecal administration of morphine for long periods has been shown to suppress the HPA axis. [5] Oltmann’s et al, reported secondary adrenal insufficiency from chronic transdermal fentanyl patch application in a 64-year-old man being treated for sciatic pain syndrome. [6] Mussig et al, reported a case of secondary adrenal failure and secondary amenorrhoea following hydromorphone treatment. [7] Dackis et al reported another case of methadone induced hypoadrenalism. [8] Miguel et al, reported a case of Tramadol-induced adrenal insufficiency in a 21-year-old female. To the best of our knowledge this is the first case report of opioid induced adrenal insufficiency in the United States. It is the first case report showing secondary adrenal insufficiency due to a prolonged combined use of hydrocodone and fentanyl.
To date, there are no guidelines for the routine monitoring of the HPA axis in patients who are on long-term opiate use. Signs and symptoms suggestive of adrenal insufficiency should prompt the physician to assess their patient’s adrenal status. [9] Chronic use of opioids appears to be associated with secondary adrenal insufficiency. This can hamper an individual’s stress response and can lead to adrenal crisis. Physicians should discuss this possibility with their patients and should discourage the long-term use of opioids.
References



