Introduction
PNETs are relatively rare and represent a heterogeneous group of tumors with varying biology behaviour and prognosis. They can be classified as functional or non functional.
Advanced PNETs remain a difficult therapeutic challenge because of high malignant potential and resistance to conventional chemotherapy. Surgery with curative intent is the mainstay of treatment for these tumors, although, there have been recent developments with promising results by using novel agents like target therapy.
We highlight an example of large PNET requiring multivisceral resection and we do a literature’s review to demonstrate that such complex operations can be achieved safely.
Case report
A 52- year old man presented with a three month history of progressive upper abdominal discomfort and constipation with intermittent rectal bleeding. There was no medical history. Clinically, he possessed a firm left upper quadrant with tender mass of 10×7 cm. Blood tests showed mildly anaemia: Hb : 10.2 g/dl. Because of recent change in his transit habits we performed a colonoscopy that revealed at the level of the splenic flexure an irregular formation without active bleeding. Endoscopic biopsies confirmed the neoplastic origin and its much suspected pancreatic origin, but they cannot distinguish between undifferentiated ductal-type adenocarcinoma and endocrine tumor. Chromogranine A (CgA) level was elevated at 2072 u/l. CT of the abdomen, showed a large pancreatic mass ( 10x7x5cm) invading the spleen and its vessels with segmental portal hypertension (PHT). The left transverse colon seemed in timely involved with the tumor (Fig.1).
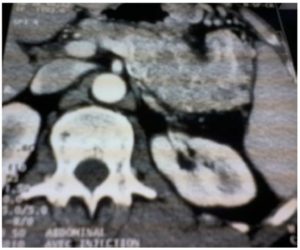
Figure 1 – a: Computed tomography showing a huge tumor of the tail of the pancreas (7 x 10 cm)
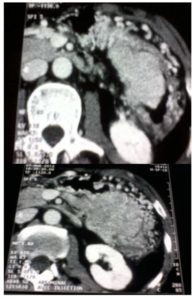
Figure 1 – b: Invasion of the left colon with segmental portal vein hypertension
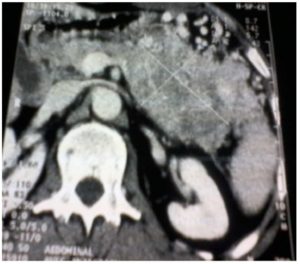
Figure 1 – C: invasion of the spleen and splenic vessels
The stomach; the portal, superior mesenteric vein (SMV), the superior mesenteric artery (SMA) and the ceoliac trunk were not involved. The liver was free of disease and there was no suspected adenopathies particularly in the ceoliac and mesenteric space. At a multidisciplinary team meeting; consensus of opinion was that the patient should be offered resection. A midline laparotomy was performed. Exploration confirmed the left pancreatic mass invaded the spleen and its vessels, the adjacent transverse left colon, the great omentum with its gross veins (segmental PHT) that enrolled the tumoral mass. There was no metastasis in the liver at the visual and manual exploration. The SMV and SMA were identified in the infracolic compartment. Following mobilisation of the splenopancreatic mass with a dissection plane along the left Gerota’s fascia, the left renal vein and left adrenal gland were not invaded. The left transverse colon was also mobilised and the short vessels were divided. We performed a bloc resection consisting of the left pancreas, the spleen, the great omentum and the left transverse colon. The neck of the pancreas was then transacted. The specimen was removed from the abdomen. (Fig.2).
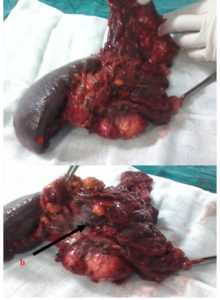
Figure 2: Operative specimen: a- En bloc resection comprising the spleen, the left pancreas and adjacent left transverse colon. b- The pancreatic tumor invading the adjacent colon (arrow)
Reconstruction involved a side-side colo-colic anastomosis. The pancreatic stump was closed with interrupted non resorbable suture (Prolen 4/0) with elective closure of the main pancreatic duct. The abdomen was drained and closed. Operative time was three hours with an estimated blood loss of 250 ml. Histopathological examination revealed a well differentiated PNET with 10 cm in diameter, with local invasion of the adjacent colon but all microscopic margins were clear (Fig.3). The mitotic rate was three mitosis per 10 HPL, and the Ki 67 proliferative index was < 2% (Fig.3).
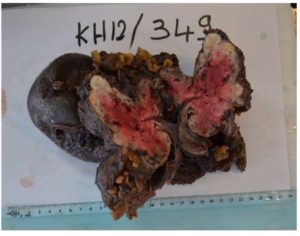
Figure 3: Macroscopic view of the specimen after formalin fixation
The post operative course was uneventful. He was discharged from hospital at 10th day. The diagnosis of well differentiated pancreatic NET grade 1 is retained. There is no indication for adjuvant chemotherapy. Follow up showed a good functional recovery from surgery. The patient remains well 16 months following resection. CT scans at 3, 6 and 12 months showed no evidence of recurrence and the Chromogranine A level is decreased with sub normalization at 16 months of follow up.
Discussion
PNETs are rare neoplasms accounting for less than 3% of all pancreatic neoplasms [1-5]. They may be categorised as functional or non functional tumors based on the characteristic clinical syndrome they cause owing to specific hormone synthesis and secretion. Except for insulinoma; PNETs are malignant in more than 50-60% of cases. In various studies, 50 to 70% of patients with advanced disease have died within 5 years of tumor progression [1, 3, 4-6].
Our case report is particular because of its mode of revelation inherent to the involvement of adjacent left transverse colon causing transit change and rectal bleeding, the segmental PVH which had complicated the surgical procedure in this advanced stage tumor; and finally; the debulking and aggressive surgery that was attempted with the hope of achieving a long term disease free- survive.
The natural history of PNETs is difficult to predict despite advances in staging, grading and classification system. To enhance clinical decision making utility, these systems have recently been rationalised by ENETs in the form of clinical guidelines for diagnosis and management [7]. It is obvious that PNETs were more favourable than exocrine cancer in terms of outcomes and should therefore be addressed differently [1, 5, 8, 9]. Surgery is the only potentially curative treatment for these tumors because chemotherapy and radiation therapy have not demonstrated any significant antitumor effects [1,5,9]. Furthermore, if all of the tumor can be removed with extensive surgery; the duration and quality of life may be greatly enhanced [9]. Multivisceral resection is defined as the resection of the pancreas and other organs not usually included in standard pancreatic operation [8]. The role of cytoreductive surgery remains controversial. Retrospective analyses have shown some survival benefit following surgery for locally advanced disease. In the retrospective study of Abou Hilal et al. [10], there has been a compiling 12 consecutive patients who underwent multivisceral en bloc resection for PNETs with a median of three additional organs resected. There were no post operative mortality and five patients experienced complications. They concluded that an aggressive approach is technically feasible and in selected patients can be achieved with low mortality and acceptable morbidity.
Resection of these advanced tumors is so desirable to ameliorate the mass effect; which can be fatal; and to reduce the incidence and severity of debilitating endocrine symptoms.
In the retrospective series published by Klein et al. [11], they analyse 41 patients who had consecutive resection for PNETs. Thirteen of whom underwent extended surgical resection in addition to pancreatic resection. There was no death in hospital stay. Patients who underwent extended resection had similar disease-specific survival to those who had pancreatic resection alone but with higher frequency complications. Furthermore, patients who had liver resection for metastases had similar disease specific survival to those without liver metastases. Other retrospective studies demonstrated the same results and concluded that conventional contraindications of surgical resection, such as SMV invasion, nodal or distant metastases, should be reconsidered in patients with advanced PNETs [12-17]. However, despite these conclusions, this evidence (as articulated in the ENETS guidelines) suffers from a heterogeneous patient/ tumor cohort and multimodality treatment strategies that make conclusions regarding aggressive surgery difficult to propose [7,11,17].
With regard to liver metastases, a number of studies have shown that combined or staged resection and small volume metastatic liver disease improves survival outcomes [5,11,15-19]. ENETS guidelines [7] suggest that resection should only be reserved if at least 90% of the volume’s tumor can be removed. In the study by TOUZIOS et al [18]; the median and 5- year survival rate were only 20 months and 25% for patients with their liver metastases treated in a non aggressive way, versus > 96 months and 72% for a group of patients who underwent hepatic resection and /or radiofrequency ablation of their liver disease. In the study by Fendrich et al [19], 27 patients with metastases from PNETs were treated surgically and a 5, 10 and actuarial 25-year survival rate of 81%, 72%, and 36% respectively, was achieved. In the setting of PENTs with unresectable liver metastases, Capruso et al [20], in their systematic review, conclude that the resection of the primary is not recommended except for selected low risk patients with life-threatening symptoms due to complications of the primary lesions. Solorzano et al [21], reported survival data for 163 patients with PNETs. Among those with localized disease, an additional survival advantage was demonstrated for patients who underwent complete resection of the primary tumor, compared to those with unresectable tumors (7.2vs 5.2 years). The management of these inoperable patients may involve a spectrum of multimodality therapy. In highly selected cases, even liver transplantation may be considered [7, 10, 21-26]. A number of biologically targeted agents targeting the VEGF and m TOR signalling pathways have recently shown promise, with recent trials showing treatment with the VEGF tyrosine kinase inhibitor SUNITINIB or the mTOR inhibitor EVEROLIMUS improves progression free survival in patients with advanced PENTs [24-28]. The high rate of somatostatine receptor expression in PNETs also provides a rationale for peptide receptor radio nucleotide therapy in patients with inoperable or metastatic disease. The most frequently used radionucletides for targeted radiotherapy are Yttrium (90Y); and Lutetium (177LU) [28-31].
Future studies will likely build on these results; further improving therapeutic options for patients with advanced PNETs.
Conclusion
In conclusion, our case report and bibliographic findings suggest that surgical debulking in patients with advanced PNETs should be increasingly considered if all gross tumors can be safely removed. This extensive surgery can be performed with acceptable mortality rate. Although long-term cure can only be achieved in a proportion of patients with malignant PNETs, significant long-term palliation can be achieved. Its use coupled with adjuvant medical treatment (Somatostatin analogues, radiolabeled somatostatin analogues, or chemotherapy) should be systematically studied. This combination may result in enhanced survival rates.
References
1. Halfdanarson TR; Rabe K.G; Rubin J; Petersen G.M (2008). Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann of Oncology, 19:1727-1733.
Publisher – Google Scholar.
2. Niederle M.B; Hack M; Kaserer K; Niederle B (2011). Gastroenteropancreatic neuroendocrine tumors: the current incidence and staging based on the WHO and European Neuroendocrine Tumor Society Classification: an anlysis based on prospectively collected parameters. Endocrine- related Cancer (2010), 17: 909-918.
Publisher – Google Scholar.
3. Sandberg A (2011). A. Pancreatic endocrine tumors. North Am J Med Sci, 3: 164-166.
Publisher – Google Scholar.
4. Mustafa E.S; Pechianu C; Iorgescu A; Hortopan M; Dima S.O; Tomulescu V et al (2012). Pathological characteristics and clinical specifications in gastropancreatic neuroendocrine tumors: A study of 68 cases. Rom J Morphol Embryol, 53: 351-355.
5. Philips S; Shah N; Vikram R; Verma S; Shanbhogue A.K.P; Prasad S.R (2012). Pancreatic endocrine neoplasm: a current update on genetics and imaging. The British Journal of Radiology, 85: 682-696.
Publisher – Google Scholar.
6. Pape U.F; Jann H; Muller-Nordhom J; Bockelbrink A; Berndt U; Willich S.N; Koch M; Rocken C; Rindi G; Wiedenmann B (2008). Prognostic Relevance of a novel TNM Classification System for Upper Gastroenterpancreatic Neuroendocrine Tumors. Cancer, 113: 256-265.
Publisher – Google Scholar.
7. Falconi M. Plakkinger; Kwekkeboom D et al (2006). Well-differentiated pancreatic non functioning tumors/ carcinoma. Neuroendocrinology, 84: 196-211.
8. Barreto SG; Shukla PJ; Shrikhande SV (2010). Tumors of the Pancreatic Body and Tail. World J Oncol, 1: 52-65.
Publisher – Google Scholar.
9. Gundara J.S; Alvardo-Bachman R; Williams N; Gananadha S; Gill A; Hugh T.J; Samra JS(2011) . Multivisceral resection of pancreatic neuroendocrine tumours: a report of two cases. WJSO, 9: 93.
Publisher – Google Scholar.
10. Abu Hilal M; Mc Phail Mark JW; Zeidan Bashar A; Jones Ceri E; Johnson Colin D; Pearce Neil W (2009). Aggressive Multivisceral Pancreatic resection for Locally Advanced Neuroendocrine Tumors. Is it worth it? JOP, 10: 276-279.
Publisher – Google Scholar.
11. Kleim M; Schrem H; Vondran FW; Krech T; Klempnauer J; Bektas H. (2012) Extended surgery for advanced pancreatic endocrine tumours. Br J Surg, 99: 88-94.
Publisher – Google Scholar.
12. Norton J.A; Kivlen M; Li M; Schneider D; Chuter T; Jensen RT (2003). Morbidity and Mortality of Aggressive Resection in Patients with Advanced Neuroendocrine Tumors. Arch Surg, 138: 859-866.
Publisher – Google Scholar.
13. Schurr PG; Strate T; Rese K. Kaifi JT; Reichelt U; Petri S; Kleinhans H; Yekebas EF; Izbicki JR. Aggressive Surgery Long-term Survival Neuroendocrine Pancreatic Tumors. An Institutional Experience. Ann Surg, 245: 273-281.
Publisher – Google Scholar.
14. Sasson AR; Hoffman JP; Ross EA; Kagan SA; Pingpank JF; Eisenberg BL. (2002) En Bloc resection for locally advanced cancer of the pancreas: is it worthwhile? J Gastrointest Surg, 6: 147-157.
Publisher – Google Scholar.
15. Pingpank JF; Hoffman JP; Sigurdson ER; Ross E; Sasson AR; Eisenberg BL.(2002) Pancreatic resection for locally advanced primary and metastatic nonpancreatic neoplasm. Am Surg, 68: 337-340.
Publisher – Google Scholar.
16. Fendrich V; Langer P; Celik I; Bartsch DK; Zielke A; Ramaswamy A; Rothmund M.(2006) An Aggressive Surgical Approach Leads to Long-term Survival in Patients with Pancreatic Endocrine Tumors. Ann Surg, 244: 845-853.
Publisher – Google Scholar.
17. Bonney GK; Gomez D; Rahman D.H; Verbeke C.S; Prasad K.R; Toogood G.J; A Lodge J.P; Menon K.V.(2008) Results Following Surgical Resection for Malignant Pancreatic Neuroendocrine Tumours. A Single Institutional Experience. JOP, 9: 19-25.
Publisher – Google Scholar.
18. Touzios J.G; Kiely J.M; Pitt S.C; Rilling W.S; Quebbeman E.J; Wilson S.D; Pitt H.A. (2005) Neuroendocrine Hepatic Metastases. Does Aggressive Management Improve Survival? Ann Surg, 241: 776-785.
Publisher – Google Scholar.
19. Fendrich V; Michi P; Habbe N; Bartsch DK. (2010). Liver-specific therapies for metastases of neuroendocrine pancreatic tumors. World J Hepatol; 2: 367-373.
Publisher – Google Scholar.
20. Capurso G; Bettini R; Rinzivillo M; Bononsegna L; Dell Fave G; Falconi M.(2011) Role of Resection of the primary Pancreatic Neuroendocrine Tumor only in Patients With Unresectable Metastatic Liver Disease: A Systematic Review. Neuroendocrinology, 93: 223-229.
Publisher – Google Scholar.
21. Solarzano CC (2001). Non functioning Iselt all Carcinoma of the Pancreas: Survival Results in a Contemporary Series of 163 Patients. Surgery, 130: 1078-1085.
Publisher – Google Scholar.
22. Cerquiera M; Machado C. (2012) Surgical Treatment of Pancreatic Endocrine Tumors in Multiple Endocrine Neoplasia type 1. Clinics, 67: 145-148.
Publisher – Google Scholar.
23. Yamazaki M; Suzuki S; Kosugi S; Okamoto T; Uchino S; Miya A; Imai T; Kaji H; Komoto I; Miura D; Yamada M; Uruno T; Horiuchi K; Sato A; Miyauchi A; Imamura M; Sakura I A; and MEN Consortium of Japan (2012). Delay in the diagnosis of multiple endocrine neoplasia type 1: typical symptoms are frequently overlooked. Advance Publication EJ 12-0071.
Publisher – Google Scholar.
24. Tonelli F; Fratini G; Nesi G; Tommasi M.S; Batignani G; Falchetti A; Brandi M L. (2006) Pancreatectomy in Multiple Endocrine Neoplasia Type-1 Related Gstrinoma and Pancreatic Endocrine Neoplsias. Ann Surg, 244: 61-70.
Publisher – Google Scholar.
25. Sarmiento JM; Heywood G; Rubin J; Llstrup DM; Nagorney DM; Que FG.(2003) Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg, 197: 29-37.
Publisher – Google Scholar.
26. Bruzoni M; Parikh P; Celis R; Are C; Ly QP; Meza JL; Sasson AR.(2009) Management of the primary tumor in patients with metastatic pancreatic Neuroendocrine tumor. A contemporary single-institution review. A, J Surg, 197: 376-81.
Publisher – Google Scholar.
27. Bonavent M; De Miquel MJ; Garcia-Carbonero R. (2012) New Targeted Agent in Gastroenteropancreatic Neuroendocrine Tumors. Target Oncol, 7: 99-106.
Publisher – Google Scholar.
28. Oberstein PE; Remotti H; Saif MW; Libutti SK.(2012) Pancreatic Neuroendocrine Tumors: Entering a New Era. Highlights from the “2012 ASCO Gastrointestinal Cancers Symposium” San Francisco, USA. January 19-21, 2012. JOP, 13: 169-173.
Google Scholar.
29. Cen P; Amato RJ.(2012) Treatment of advanced pancreatic neuroendocrine tumors: potential role of Everolimus. Onco Targets and Therapy, 5: 217-224.
Google Scholar.
30. Saif MW; Ng J; Chang B; Russo S. (2012) Is There a Role of Radiotherapy in the Management of Pancreatic Neuroendocrine Tumors? Highlight from the “2012 ASCO Gastrointestinal Cancers Symposium” San Francisco, USA. January 19-21, 2012. JOP, 13: 174-176.
Google Scholar.
31. Dimou AT; Syrigos KN; Saif MW. (2010) Neuroendocrine Tumors of the Pancreas: What’s new? Highlight from the “2010 ASCO Gastrointestinal Cancers Symposium” Orlondo, FL,, USA. January 22-24, 2010. JOP, 11: 135-138.







