Introduction
Hepatitis C is under diagnosed and affects 1.8% of North Americans and 5.4% of Kuwaitis [1]. Chronic hepatitis C virus (HCV) infection is attributed as a cause of circulating mixed cryoglobulinemia and immune mediated extrahepatic complications [2]. There are multiple reported cases of acute kidney injury secondary to cyroglobulonemia and glormerulonephritis. Similarly, reports of massive pulmonary hemorrhage have been documented in patients suffering from above [3].
Treatment options for cryoglobulinemia and pulmonary-renal syndrome are limited, and randomized controlled trials are reasonably lacking. The consistently used regimen includes plasmapheresis with high-dose prednisolone and cyclophosphamide along with treatment of the underlying cause. Rituximab, popularly recognized as a monoclonal anti-CD20 antibody, has been the recommended treatment modality in a number of autoimmune diseases [4].
This is case report of a patient presenting with hepatitis C, mixed cryoglobulinemia and pulmonary hemorrhage resistant to standard treatment that experienced HCV reactivation and fulminant hepatitis upon receiving Rituximab (RTX) therapy.
Case Report
We report a 56-year-old Kuwaiti woman, known to have diabetes mellitus (DM) for 7 years and hypertension for 10 years. The patient has chronic hepatitis C, first discovered in 2005, for which she received interferon and ribavirin for one year. Treatment was discontinued due to lack of response. The genotype of the HCV strain was 1b.
She arrived at the medical outpatient room with a 60-day history of progressive abdominal distension, later on associated with a dry cough and shortness of breath of 30 days duration.
The patient had mild orthopnea attributed it to abdominal distension, but neither paroxysmal nocturnal dyspnea nor chest pain. She had 4 uncomplicated pregnancies. A detailed review of other possible system involvement was unremarkable. The patient had not received blood transfusion and was not taking any medications. The patient didn’t have any social history of medical relevance (including tobacco, alcohol, or illicit drugs abuse). The patient’s family history was also unremarkable. She was not employed outside the home.
On admission, the patient was not dyspnic. Vitals included a temperature of 37.6°C, pulse of 110 beats per min and respiratory rate of 24 breaths per min. Oxygen saturation was at 100% on room air. The patient’s cardiac examination was normal and no added sounds were heard. The patient’s lungs revealed bilateral basal crepitations. She had clinically significant ascites and marked lower limb edema.
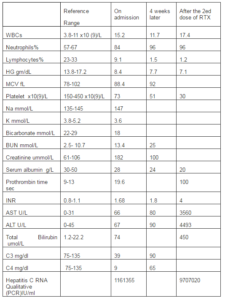
Laboratory tests are shown in table 1. A chest radiograph revealed no abnormalities.
Table 1: Laboratory Tests
Therapeutic paracentesis was performed and removed 5 liters of clear fluid and received 40 g albumin. Serum —Ascites Albumin Gradient was 15, and no leukocytes were detected.
The cough and dyspnea progressed over the next 3 days. Along several hours, the patient’s respiratory function had rapidly declined. She starting coughing sputum with frank blood, and trachea was rapidly intubated due to impending hypoxic respiratory failure. Copious bloody secretions were pouring out from the endotracheal tube.
Bronchoscopy was remarkable for bloody fluid on successive bronchoalveolar lavages (BAL) of the right-lower lobe to lesser extent in the left lung, consistent with diffuse alveolar hemorrhage. Lavage fluid was predominantly polymorph nuclear leukocytes (76% of 850 leukocytes/ ul). The microscopic examination of the aspirate revealed leukocytes, without organisms; stains for acid-fast bacilli, and viruses testing were negative. A cytological examination showed evidence of numerous hemosiderin-laden pulmonary macrophages, but no malignant cells. A right lung biopsy showed neutrophilic vasculitis with focal necrosis and mild fibrosis.
The peripheral blood smear revealed no fragmented erythrocytes and no other abnormal findings. The blood cultures were sterile; the patient received ceftriaxone.
A computed tomography (CT) scan of the chest revealed scattered bilateral alveolar opacities (Fig. 1).
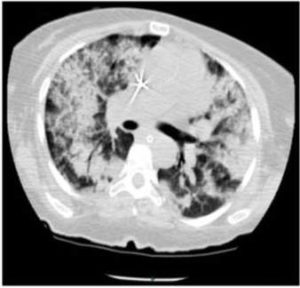
Fig. 1. CT of Chest Showing Diffuse Bilateral Alveolar Infiltrates
In addition to that, serological markers for antinuclear antibodies, antiglomerular basement membrane, ELISA for proteinase-3 (PR3) and myeloperoxidase (MPO), and cryoglobulins were negative. Also, test for hepatitis B surface antibody, hepatitis B surface antigen and HIV were also negative. Testing for hepatitis C antibody was positive, and the viral load was 1161355 IU per milliliter. Testing was negative for pneumocystis jiroveci.
A diagnosis of pulmonary—renal disease and was suggested and the patient was managed with methylprednisolone (1 gram intravenously, once daily for 3 days). Plasmapheresis was initiated using fresh-frozen plasma. The pulmonary hemorrhage subsided, and hemodialysis was initiated because the patient became oliguric with increasing in creatinine level. Steroids were slowly tapered over the next 4 weeks to a dose of 15 mg of prednisone per day. The plasmapheresis was continued at the same dose for 12 courses every 36 hours, hand in hand with hemodialysis. This led to an improvement in the patient’s respiratory and renal function. Pulmonary hemorrhage recurred when plasmapheresis was stopped, the C3 and C4 level dropped. Cryoglobulin measurement was positive when repeated and Prednisone dose was consequently increased along with plasmapheresis.
Blood and sputum cultures revealed the presence of acinobacter bumanii and klebsiella which responded to IV administered ciprofloxacin and cefepime.
RTX was started on the 3th week of plasmophoresis. After a second dose of RTX, the respiratory status and liver function test improved and the trachea was extubated.
The 3rd dose of RTX was not administered because the patient’s serum alanine aminotransferase (ALT) concentration raised to 4503 U per liter (it was 90U per liter at the time of 2nd dose of RTX). Icterus recurred, progressive encephalopathy and coagulopathy developed. There was a shut-down of acute renal functions, which were restarted using CRRT. The trachea was reintubated after 4 weeks of starting RTX. The patient’s peak serum bilirubin concentration was 256 µmol/L, and the longest prothrombin time was 100 seconds. Activated partial thromboplastin time (aPTT) was also prolonged. Additional ascites were observed and were drained multiple times; the INR reached 4, and hypoglycemia and MELD scores were 36. The patient died in hepatic coma and fulminant hepatitis.
Discussion
The patient in this report presented with progressive respiratory distress and hemoptysis. Alveolar hemorrhage was indicated because of the severe anemia suggested acute blood loss into lung tissue and signs observed by imaging and bronchoscopy. No microangiopathic features were noted. She presented with a combination of pulmonary hemorrhage and acute renal failure suggestive small-vessel vasculitis. Positive-serologic studies for hepatitis C and resistance to antiviral treatment posed a question: Is the patient’s acute presentation directly related to the hepatitis C virus infection? Bearing in mind that infection with HCV might explain all of the life-threatening autoimmune complications of her illness were critical to identifying proper therapy options, with least side effects.
Cryoglobulins are immunoglobulins that precipitate in cold temperatures and redissolve when heated. They are found to be associated with chronic infections, especially HIV infection and hepatitis C, Lymphoproliferative disorders, systemic lupus erythematosus, and rheumatoid arthritis [5].It is important to recognize that false negative serum cryoglobulin tests are common [6], to avoid premature rejection of the diagnosis. The test needs careful dealing with sample to obtain a true result.
Cryoglobulinemia is frequently present in up to 40% HCV- positive patients; however, vasculitis related to cryoglobins is observed only in 2 to 10% of the patients [2]. The cryoglobulinemic vasculitis have wide range of clinical manifestations, ranging from mild sporadic palpable prupura in 80% of cases, nerve involvement in 50% of cases, glommerulonphritis in 30% of cases to rare life-threatening hemorrhagic alveolitis and cardiomyopathy [7].
Immunosuppressive agents are needed to treat life-threatening cryoglobulinemic vasculitis in HCV-positive patients; on the other hand, immunosuppressive agents may worsen the underlying infection. Long term immunosuppression, as in transplant recipients with positive HCV, may increase the liver disease progression; however, short courses of corticosteroids have no effect on liver status [8, 9]. For patients in whom antiviral therapy is not applicable, or who are nonresponders, as in our patient, treatment should be modified according to the severity of vasculitis complication. The effectiveness of antiviral treatment in cryoglobulinemic vasculitis with HCV-positive was shown in 40 to 60% of cases with frequent relapses after completion of antiviral therapy [10]. Using interferon α and ribavirin therapy to control viremia resulted in resolution of cryoglobulins in patients with hepatitis C induced- glomerulonephritis in small scale studies [11]. Antiviral treatment was not given to this patient because of the previous failure of antiviral therapy and presence of decompensated liver cirrhosis.
B-cell depletion therapy is considered to be safe in rheumatoid patients with concomitant hepatitis [12].RTX has also been used for the treatment of HCV-associated cryoglobinemia and its complication in patients with lymphoma [13]. Deterioration in liver function has been shown to occur when RTX is used in adjunction with other immunosuppressive agents rather than as a standalone agent. RTX is a monoclonal Anti-CD20 antigen, which is selectively seen in B cells. The purpose behind RTX treatment is that in cryoglobulinemic vasculitis, cells with CD 20 markers are abundant, and play an important role in producing cryoglobulin [14].
It was shown that RTX is highly effective when HCV- induced cryoglobulinemic- vasculitis was refractory to antiviral regimens or patients with recurrent vasculitis after initial therapy [15, 16] and that combining of RTX with antiviral regimen may produce better clinical response [17]. More recently RTX was shown to be more efficient than traditional immunosuppression treatments [18], with some variation according to the different manifestations of cryoglobulin vasculitis. Based on meta-analyses, the response rate to RTX for patients not responding to antivirals has been reported to be > 80% [19].
According to the fragmentary data available, in oncohematological patients, RTX-based chemotherapy can increase HCV replication [20], a biological event that has been associated with the development of Acute Liver toxicity in some studies [21]. RTX-based chemotherapy can initiate an increase in HCV expression in hepatic cells, which becomes targets for a cell-mediated immune reaction, explaining the hepatic toxicity [22]. In a retrospective study of 131patients with HCV infection and NHL treated with RTX and prednisolone-based chemotherapy, Ennishi et al. [21] describe a hepatic flare in 36 patients (27%). In 10% of the cases, the hepatic toxicity was severe and some patients died of fulminant hepatitis.
Studies have reported a frequent hepatic decompensation in patients receiving combination of high-dose corticosteroids and RTX [20]. This outcome is most likely due to the cumulative effect of both drugs in increasing HCV replication, as well as the impairment of antibody production by RTX [22] and facilitate of HCV entry to the hepatocytes by corticosteroids.
This case represents a rare autoimmune complication of a common disease. Treatment should be modified according to the severity of vasculitis manifestations, in the absence of clear guidelines and evidence base medicine.
Our case report sheds light on the need for further exploration of new therapeutic agents in the treatment of resistant cryoglobulinaemia associated with HCV. There has been no study which has yet examined the efficacy of rituximab in mixed cryoglobulinemia prospectively. This is further complicated by the low number of patients in individual centers, making randomized controlled trials seemingly impossible. Consequently, the current literature is restricted to case reports and case series necessitating prospective studies to understand the role of RTX. Resistant cryoglobulinemia is a life-threatening condition, whose optimal management remains elusive.
References
1] Fallahnian, F. & Najaf, A. (2011). “Epidemiology of Hepatitis C in the Middle East,” Saudi Journal of Kidney Diseases and Transplantation, 22(1): 1-9.
Publisher – Google Scholar
2] Cacoub, P. et al. (1999). “Extrahepatic Manifestations of Chronic Hepatitis C,” Arthritis & Rheumatism, 42:2204-12.
Publisher – Google Scholar
3] Gomez-Tello, V. et al. (1999). “Diffuse Recidivant Alveolar Hemorrhage in a Patient with Hepatitis C Virus-Related Mixed Cryoglobulinemia,” Intensive Care Medicine, 25:319-22.
Publisher – Google Scholar
4] Cacoub, P., Costedoat-Chalumeau, N., Lidove, O. & Alric, L. (2002). “Cryoglobulinemia Vasculitis,” Current Opinion in Rheumatology, 14:29- 35.
Publisher – Google Scholar
5] Ramos-Casals, M. et al. (2010). “Off-Label Use of Rituximab in 196 Patients with Severe, Refractory Systemic Autoimmune Diseases,” Clinical and Experimental Rheumatology, 28:468—476.
Publisher – Google Scholar
6] Musset, L. et al. (1992). “Characterization of Cryoglobulins by Immunoblotting,” Clin Chem, 38:798- 802.
Publisher – Google Scholar
7] Vassilopoulos, D. & Calabrese, L. H. (2002). “Hepatitis C Virus Infection and Vasculitis: Implications of Antiviral and Immunosuppressive Therapies,” Arthritis & Rheumatism; 46:585-97.
Publisher – Google Scholar
8] Calleja, J. L., Albillos, A., Cacho, G., Iborra, J., Abreu, L. & Escartin, P. (1996). “Interferon and Prednisone Therapy in Chronic Hepatitis C with Non-Organ Specific Antibodies,” Journal of Hepatology, 24:308-12.
Publisher – Google Scholar
9] Benhamou, Y. et al. (1999). “Liver Fibrosis Progression in Human Immunodeficiency Virus and Hepatitis C Virus Coinfected Patients,” Hepatology, 30:1054-8.
Publisher – Google Scholar
10] Misiani, R. et al. (1994). “Interferon Alfa-2a Therapy in Cryoglobulinemia Associated with Hepatitis C Virus,” New England Journal of Medicine, 330:751-6.
Publisher – Google Scholar
11] Sabry, A. A. et al. (2002). “Effect of Combination Therapy (Ribavirin and Interferon) in HCV-Related Glomerulopathy,”Nephrology Dialysis Transplantation, 17:1924-30.
Publisher – Google Scholar
12] Saag, K. G. et al (2008). “American College of Rheumatology 2008 Recommendations for the Use of Non- Biologic and Biologic Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis,” Arthritis and Rheumatism, 59:762e84
Publisher – Google Scholar
13] Lin, K.- M. et al. (2013). “Rituximab-Induced Hepatitis C Virus Reactivation in Rheumatoid Arthritis,” Journal of Microbiology, Immunology and Infection 46, 65e67
Publisher – Google Scholar
14] Fukushima, N. et al. (2009). “Retrospective and Prospective Studies of Hepatitis B Virus Reactivation in Malignant Lymphoma with Occult HBV Carrier,” Annals of Oncology, vol. 20, 12, 2013—2017.
Publisher – Google Scholar
15] Sansonno, D. et al. (2003). “Treatment of Mixed Cryoglobulinemia Resistant to Interferon α with an Anti-CD20Monoclonal Antibody,” Blood, vol. 101, 10, 3818—3826.
Publisher – Google Scholar
16] Zaja, F. et al. (2003). “Efficacy and Safety of Rituximab in Type II Mixed Cryoglobulinemia,” Blood, vol. 101, 10, 3827—3834.
Publisher – Google Scholar
17] Saadoun, D. et al. (2010). “Rituximab Plus Peg-Interferon-α/Ribavirin Compared with Peg-Interferon- α/Ribavirin in Hepatitis C-Related Mixed Cryoglobulinemia,” Blood, vol. 116, 3, 326—334.
Publisher – Google Scholar
18] Sneller, M. C., Hu, Z. & Langford, C. A. (2012). “A Randomized Controlled Trial of Rituximab Following Failure of Antiviral Therapy for Hepatitis C Virus-Associated Cryoglobulinemic Vasculitis,” Arthritis and Rheumatism, vol. 64, 3, 835— 842.
Publisher – Google Scholar
19] Cacoub, P., Delluc, A., Saadoun, D., Landau, D. A. & Sene, D. (2008). “Anti-CD20 Monoclonal Antibody (Rituximab) Treatment for Cryoglobulinemia Vasculitis: Where Do We Stand?,” Annals of the Rheumatic Diseases, vol. 67, 3, 283—287.
Publisher – Google Scholar
20] Aksoy, S., Abali, H., Kilickap, S., Erman, M. & Kars, A. (2006). “Accelerated Hepatitis C Virus Replication with Rituximab Treatment in a Non-Hodgkin’s Lymphoma Patient,” Clinical and Laboratory Haematology, vol. 28, 3, 211—214.
Publisher – Google Scholar
21] Ennishi, D. et al. (2010). “Hepatic Toxicity and Prognosis in Hepatitis C Virus-Infected Patients with Diffuse Large B-Cell Lymphoma Treated with Rituximab-Containing Chemotherapy Regimens: A Japanese Multicenter Analysis,” Blood, vol. 116, 24, 5119—5125.
Publisher – Google Scholar
22] Sagnelli, E., Pisaturo, M., Sagnelli, C. & Coppola, N. (2012). “Rituximab-Based Treatment, HCV Replication, and Hepatic Flares,” Clinical and Developmental Immunology, Volume 2012, 1-5
Publisher – Google Scholar




