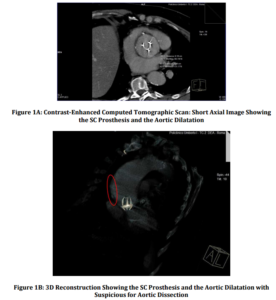
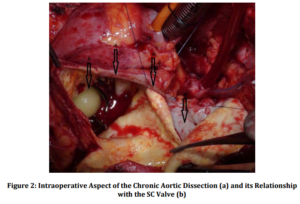
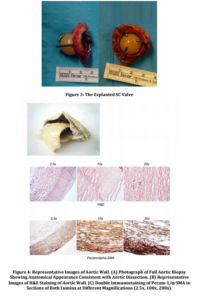
Discussion
The SC is a first generation heart valve prosthesis that has been successfully implanted in a large number of patients. Functional characteristics of this prosthesis are similar to the Starr Edwards (SE) valve with several important differences related to the structural conformation that determine its unique flow performance making this valve the first “full flow” ball prosthesis. The SC valve has, in fact, an additional titanium cage on the bottom that hold the silicon ball in the valve ring during diastole determining a slight regurgitation around the ball with a consequent self-washing effect that prevents the formation of thrombotic material2. Moreover, the two cages are designed with opened struts limiting the problem of the ball variance, and consenting to use a poppet with a diameter similar to the native valve orifice. The ball is similar to the one adopted in the SE valve and is manufactured using silicon rubber. Even if the lipid absorption by the ball is a possible phenomena, the different silicon’s curing method and the open double caged design of the SC valve partially solved the complications related to the potential ball deformation yielding lower thromboembolism rate and better long-term results1, 2. Different factors have been recognized to positively affect ball-valve durability and freedom from thromboembolic events. Lund et al., identified a valve size index of 13 mm/m2 or less as an incremental risk factor for late mortality, bleeding, and other valve-related complications in patients with SE valve1. Additional factors that have been associated to a longer durability are represented by an age over 50 years at the time of operation, aortic stenosis and ascending aorta dilatation for the favorable effect on pressure gradients and turbulent stress5. Few studies have already confirmed the durability of this valve for more than 25 years in aortic position, and a recent report described an echocardiographic functionally normal SC aortic prosthesis after 40 years6. Despite the evidence of early pannus formation around the struts, thromboembolic events associated with this valve in aortic position have been reported with very low rates, even without permanent anticoagulation therapy,2, 7.
In our case the valve didn’t show structural abnormalities such as ball variance and the poppet appeared smooth with neither alteration of the circular shape nor macroscopic evident lipid absorption. Surprisingly, even if pannus formation around the struts has been reported as a routine complication7, we did not detect either pannus or thrombotic apposition around the valve struts possibly because of the self-washing effect, and the distance of the valve struts from the aortic walls. Blood flow dynamics in ball caged valves might have a significant influence on blood rheology and a great impact in aortic wall degeneration. Thus, it is possible to speculate that flow dynamics imposed by the configuration of the prosthesis causes lateralization of forward flow resulting in blood turbulence and cavitation thus increasing the risk of hemolysis and thromboembolic complications. In our patient moreover, we hypothesized that these lateralized flow characteristics may have contributed to a prolonged increased wall stress, playing a role in the determinism of a progressive aortic dilatation.8 To our knowledge, this case represents the longest reported follow up of functionally normal explanted SC aortic valve, confirming the good features of its ultra-structural conformation, but highlighting that its hemodynamic characteristics were possibly cause of the aortic pathology.
References
1. Lund, O., Pilegaard, H. K., Ilkjaer, L. B., Nielsen, S. L., Arildsen, H. & Albrechtsen, O. K. (1999). “Performance profile of the Starr-Edwards Aortic Cloth Covered Valve, Track Valve, and Silastic Ball Valve,” European Journal of Cardiothoracic Surgery, 16(4):403-13
Publisher – Google Scholar
2. Stormer, B., Mendling, W., Kohler, J. et al. (1976). “Comparative Study of in Vitro Flow Characteristics between a Human Aortic Valve and a Designed Aortic Valve and Six Corresponding Types of Prosthetic Heart Valves,” EuropeanSurgical Research, 8(2):117-31
Publisher – Google Scholar
3. Head, S. J., Ko, J., Singh, R., Roberts, W. C. & Mack, M. J. (2011). “43.4-Year Durability of a Smeloff-Cutter Ball Caged Mitral Valve,” The Annals of Thoracic Surgery, 91(2):606-8
Publisher – Google Scholar
4. Carnevale, D., Lembo, G. & Frati, G. (2011). “Chronic Type A Aortic Dissection: Could Surgical Intervention Be Guided by Molecular Markers?,” Journal of Cellular and Molecular Medicine, 15(7):1615-9
Publisher – Google Scholar
5. Nygaard, H., Paulsen, P. K., Hasenkam, J. M. et al. (1994). “Turbulent Stresses Downstream of Three Mechanical Aortic Valve Prostheses in Human Beings,” The Journal of Thoracic and Cardiovascular Surgery, 107(2):438-46
Publisher – Google Scholar
6. Suezawa, T., Morimoto, T., Jinno, T. et al. (2008). “Forty-Year Survival with Smeloff-Cutter and Starr-Edwards Prostheses,” The Annals of Thoracic Surgery, 85(3):14-6
Publisher – Google Scholar
7. Gometza, B. & Duran, C. M. G. (1995). “Ball Valve (Smeloff-Cutter) Aortic Valve Replacement without Anticoagulation,” The Annals of Thoracic Surgery, 60(5):1312-16
Publisher – Google Scholar
8. Cohn, L. H. (2007). Cardiac Surgery in the Adult, McGraw-Hill (ed), ISBN: 0071469133
Publisher