Discussion
About only 5 percent of patients with HSP eventually develop end-stage renal disease, but most of the patients with HSP resolve completely without any therapy [2]. The classifications of both Haas and Lee and colleagues are most widely used, but each has some limitations as a prognostic indicator [3]. As the prognosis is said to be poor when rapidly progressive glomerulonephritis is complicated, and prednisolone and various immunosuppressive agents are often used in the presence of severe renal involvement. Reported immunosuppressive agents include cyclosporine, cyclophosphamide, and azathioprine. IVIG and plasmapheresis have been also reported [1]. However, no standard regimen has been established. These facts may suggest that there are various causes of AKI in HSP and the renal prognosis depends on its underlying etiologies.
Combination of prednisolone and MZR is considered as one of the standard therapies of lupus nephritis and nephrotic syndrome. In such cases, prednisolone is often used as an anti-inflammatory drug and MZR as an immunosuppressive agent. The characteristics of MZR are the lack of oncogenicity and the association with a low incidence of myelosuppression and hepatotoxicity [4]. Recently, Nishioka et al. have reported the usefulness of MZR in maintenance therapy of ANCA-related vasculitis [5]. Two cases of HSP successfully treated by PSL and MZR have been also reported [6, 7]. Additive MZR administration in our case might decrease his circulating and depositing IgA effectively and improve sustained hematuria and fecal occult blood. We need to acknowledge the possibility of remission in natural course. However, clinical improvement and time course of hematuria and fecal occult blood after the administration of MZR was very suggestive (Figure 1(b)).
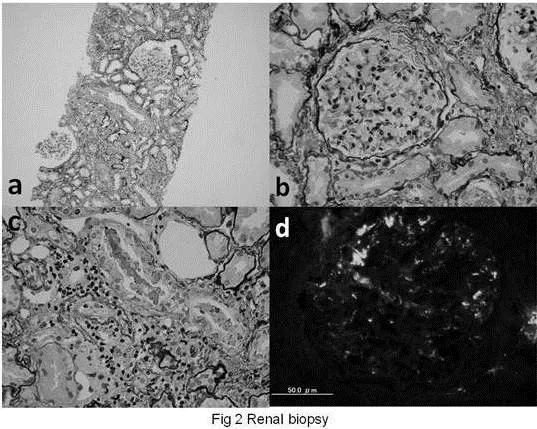
According to the literature, the histopathology of HSP nephritis is that of IgA nephropathy. It is said that the prognostic factors associated with acute kidney injury include massive cellular crescent formation. In our case, we could not find any cellular crescent formation or severe IgA depositions on mesangial cells and capillary loops. Several hyalinized glomeruli and Bowman’s capsules might be a ruin of cured crescent, but, usually, healing process is not occurred so early. And besides, we could not find any residue of vasculitis in the stroma, but some small foci of lymphoid cell accumulation in the hyalinized stroma. On the other hand, there were some erythrocyte-casts in distal tubules, which suggested a remnant of macrohematuria of glomerular origin. The pathological findings rather suggest erythrocyte-casts occlusion in distal tubules, than severe mesangial cell proliferation, IgA depositions, or vasculitis.
Recently, AKI during warfarin therapy has been reported [8]. The warfarin-related nephropathy is associated with acute tubular injury and glomerular hemorrhage, caused by erythrocytes in Bowman space and occlusive erythrocytes-casts in tubules. These pathological findings and occlusive mechanism of AKI are very similar to those of our HSP patient. In our case, massive gross hematuria was supposed to make numerous erythrocytes-casts in tubules and may play important role in the development of his renal manifestation. However, we need to acknowledge that no one knows the precise impact of erythrocytes-casts occlusion on the pathogenesis of AKI in this case. Previously, it had been reported that erythrocyte-casts occlusion or hemoglobin casts in tubules might induce AKI. However, recent studies suggested that the main etiology is rather the direct tubular toxicity of hemoglobin, heme, iron, or other molecules released from erythrocytes. It is said that the heme group of hemoglobin decreases nitric oxide availability, promoting intrarenal vasoconstriction and ischemia. On the other hand, lipid peroxidation and physical obstruction of the renal tubule with hemoglobin precipitation are also promoted by cell-free hemoglobin under acidic conditions [9]. These facts suggest that not the amount of erythrocytes-casts occluded in tubules but the severity of the tubular toxicity produced by erythrocytes-casts is more important. In our case, there were erythrocytes-casts in distal tubules, and some small foci of lymphoid cell accumulation in the hyalinized stroma, which might show the existence of more occluded erythrocytes-casts during AKI in acute phase. Considering these underlying mechanisms, aggressive hydration may be justified to prevent and wash out erythrocyte-casts formation, not only immunosuppressive treatment.
In conclusion, we reported a case of Henoch-Schönlein purpura with acute kidney injury accompanied by severe gross hematuria and erythrocyte-casts occlusion in distal tubules. We should consider occlusive erythrocyte-casts as one of the causes of AKI when we treat HSP patients with severe gross hematuria.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
1. Davin, J.- C. (2011). “Henoch-Schönlein Purpura Nephritis: Pathophysiology, Treatment, and Future Strategy,” Clinical Journal of the American Society of Nephrology March 2011 6: (3) 679-689.
Publisher – Google Scholar
2. Goldstein, A. R., White, R. H. R., Akuse, R. & Chantler, C. (1992). “Long-Term Follow-Up of Childhood Henoch-Schönlein Nephritis,” Lancet 1992 Feb 1; 339(8788): 280-2.
Publisher – Google Scholar
3. Feehally, J. (2001). “Predicting Prognosis in IgA Nephropathy,” American Journal of Kidney Diseases 2001 Oct; 38(4): 881-3.
Publisher – Google Scholar
4. Kawasaki, Y. (2009). “Mizoribine: A New Approach in the Treatment of Renal Disease,” Clinical and Developmental Immunology 2009: 681482.
Publisher – Google Scholar
5. Nishioka, Y., Horita, Y., Tadokoro, M., Taura, K., Suyama, N., Miyazaki, M. et al. (2006). “Mizoribine Induces Remission of Relapsed ANCA-associated Renal Vasculitis,” Nephrology Dialysis Transplantation 2006 Apr; 21(4): 1087-8.Publisher – Google Scholar
6. Kawasaki, Y., Suyama, K., Matsumoto, A., Takano, K., Hashimoto, K., Suzuki, S. et al. (2007). “Efficacy of Tonsillectomy Plus Methylprednisolone Pulse Therapy for a Child with Henoch-Schoenlein Purpura Nephritis,” The Tohoku Journal of Experimental Medicine 2007 Mar; 211(3): 291-5
Publisher – Google Scholar
7. Kawakami, T., Shirai, S. Kimura, K. & Soma, Y. (2010). “Successful Use of Mizoribine to Treat Recurrent Corticosteroid-Resistant Palpable Purpura in a Patient with Henoch-Schonlein Purpura Nephritis,” Archives of Dermatolology 2010 Feb; 146(2):212-3.
Publisher – Google Scholar
8. Brodsky, S. V., Satoskar, A., Chen, J., Nadasdy, G., Eagen, J. W., Hamirani, M. et al. (2009). “Acute Kidney Injury during Warfarin Therapy Associated with Obstructive Tubular Red Blood Cell Casts: A Report of 9 Cases,” American Journal of Kidney Diseases 2009 Dec; 54(6):1121-6.
Publisher – Google Scholar
9. Moreno, J. A., Martín-Cleary, C., Gutiérrez, E., Toldos, O., Blanco-Colio, L. M., Praga, M., Ortiz, A. & Egido, J. (2012). “AKI Associated with Macroscopic Glomerular Hematuria: Clinical and Pathophysiologic Consequences,” Clinical Journal of the American Society of Nephrology 2012 Jan; 7(1):175-84.
Publisher – Google Scholar