Introduction
Chondrosarcoma are a heterogenous group of primary malignant bone tumours, and are the third most common primary malignant bone tumour (Dorfman and Czerniak, 1995). Intracranial chondrosarcomas however are a rare entity, and account for only 0.15% of all intracranial tumours (Bloch et al., 2009, Bloch and Parsa, 2013, Bloch et al., 2010).
The median survival time for patients with intracranial chondrosarcoma has been reported to be approximately 24 months with 11.5% 5-year mortality (Bloch et al., 2009). Whilst this is consistent with previous studies (Oghalai et al., 2005, Colli and Al-Mefty, 2001), the largest study of intracranial chondrosarcoma, by Rosenberg et al., 1999, demonstrates a 5-year survival of 99% when surgery is combined with adjuvant radiotherapy (Rosenberg et al., 1999).
Intracranial Chondrosarcomas arise predominantly at the base of the skull with the most common location being the clivus (32%) followed by the temporo-occipital junction (Bloch et al., 2009, Khan et al., 2013). The predilection for the skull base is thought to be related to the difference in bone development as endochondral ossification predominates in the basilar structures of the skull compared to intramembranous ossification in other areas (Bloch and Parsa, 2013). Chondrosarcomas arise from resting chondrocytes found in areas of mature endochondral ossification. However competing theories of their origin suggest that they may originate from remnants of functional mesenchymal cells involved in the embryogenesis of the skull base, or even the metastasis of mature fibroblasts (Bloch et al., 2009).
Given the rarity of these tumours, there is significant variability in the therapeutic approach, without conclusive evidence elucidating an evidence-based approach (Bloch and Parsa, 2013). This case report describes a unique clinical experience of this rare entity, the use of an extended endoscopic endonasal approach (EEA) to the surgical management of a clival tumour and finally the use of a naso-septal flap as a reconstructive method. Overall this report adds support to the use of a minimally invasive approach for the resection of posterior clival lesions with minimal morbidity, fast recovery and reduced hospital stay.
Case Report
A 27 year old female presented with a progressive clival mass. She initially presented in 2010 with headache and diplopia, and was subsequently found to have a petroclival mass (Figure 1). A surgical resection was undertaken at the time via a frontotemporal craniotomy. No adjuvant radiotherapy was offered due to patient’s concern. Despite a detected recurrence in 2011, conservative management was chosen by the patient due to her asymptomatic status.
In 2013 her tumour had doubled in size, with increasing headache and diplopia. An MRI revealed a cystic mass measuring 21mm x 9mm in the mid-clival region (Figure 2). The tumour was right para-midline, but displaced the basilar artery to the left, with mild-distortion of the pons.
Operation
The patient underwent a combined endoscopic endonasal resection of the clival mass. Stereotactic registration was performed utilising fused CT and MRI skull base sequences.
A standard binasal approach was utilised with elevation of a vascularised nasoseptal flap. The posterior half of the nasal septum was removed to widely expose the sphenoid rostrum down to the inferior clivus before proceeding to wide sphenoidectomies and partial posterior ethmoidectomies.
The sella floor and clival recess were identified and the clivus drilled out to the inferior extent of the tumour location. The vidian nerve and artery were identified enabling horizontal and
vertical carotid localisation. The clival tumour was exposed and radically debulked, with exposure laterally to Dorello’s canal. The dural leaves were separated and resected with tumour enabling clear margins on histopathological frozen section. The basilar artery and perforators were identified and seen displaced to the left. Having resected the tumour to clear margins, a multilayered reconstruction technique was utilised with use of the previously raised naso-septal flap to reconstruct the defect. Duragen (Integra Life Sciences, Plainsboro, NJ) was used as an inlay repair, the flap as an overlay and secured with Tisseel (Baxter Healthcare Corp., Deerfield, IL). Gelfoam was packed against the reconstruction to ensure no dead space and a Foley’s balloon catheter was then inserted and inflated to maintain the gelfoam.
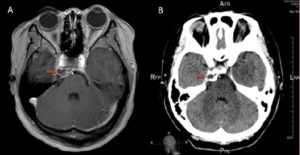
Figure 1A – MRI and CT Images taken on 29/06/10 showing initial tumour. Figure 1A is an Axial T1-weighted post-contrast MRI which reveals a right lobulated petroclival mass (red arrows) measuring 20mm x 9mm x 11mm in size. It is slightly hypotense on T1 with a ring of variable thickness, however it hypertense on T2-weighted imaging. Post-contrast there is a lobulated mass with rim enhancement and minor linear internal enhancement. It extends to the left of the midline with minor mass effect on the prepontine cistern, temporal lobe and basilar artery. The mass extends inferolaterally to the tip of the petrous apex, and abuts the posterior cavernous portion of the right internal carotid artery, however this vessel is patent with no deformation noted. There is also minor attenuation of the medial portion of the right Meckel’s cave by the mass, however, it does not appear to impede the trigeminal nerve. The optic nerve and chiams are also clear.
Figure 1B is an Axial Post-contrast CT on 29/06/10. This reveals a soft tissue mass consistent with a cartilaginous tumour situated between the petrous apex and the clivus. Calcification is seen within the periphery of the lesion, however no definitive contrast enhancement can be seen. There is erosion of the adjacent petrous apex and, to a lesser, extent part of the adjacent clivus.

Figure 2: Axial T1-weighted post contrast MRI taken on 21/05/13 showing recurrence of tumour. This reveals a lobulated mass measuring 21mm x 9mm in size located in the posterior sellar area of the anterior clival region. The mass is predominantly mid-line with some extension to the right with some mass effect displacing the basilar artery. The mass shows intense heterogeneous enhancement with associated dural thickening and enhancement. There is some associated distortion of the anterior pons (brown arrow), whilst the pituitary gland, infundibulum and the chiasm are normal.
Post-operative Course
Postoperatively the patient had an uncomplicated recovery, with resolution of pre-operative diplopia and headaches. No CSF leak occurred. The foley catheter was removed after 48 hrs and the patient discharged five days post operatively. Histopathological analysis confirmed a low grade chondrosarcoma (Figure 3).
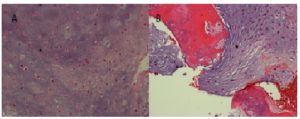
Figure 3: Histopathology: H&E stain showing a low grade chondrosarcoma (A) with bone involvement (B)
A post-operative MRI confirmed complete resection of the chondrosarcoma (Figure 4).
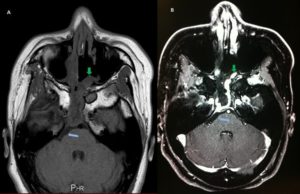
Figure 4: Day 1 Post-operative T1-weighted Axial MRI. A is without contrast whilst B is post-contrast. This reveals complete excision of the clival mass previously noted with excellent decompression (blue arrows). There is some post-operative enhancement of the sphenoid sinus region along with a thin rim of dural enhancement along the area of previous surgery extending downwards towards the inferior clivus. The nasoseptal flap is also visible (green arrows).
The patient underwent focal stereotactic radiotherapy delivered via Volumetric-Modulated Arc Therapy (VMAT), starting 6 weeks post-operatively. Thirty-four fractions of radiotherapy, with a total dose of 61.20Gray, were delivered over a 7 week period.
At 3 months post-operatively, and 3-weeks post-completion of her radiotherapy treatment the patient had nil neurological deficits or ongoing side effects. Her original symptoms had completely resolved. She was planned for 6-monthly progress MRI.
Discussion
Chondrosarcomas
Chondrosarcomas typically grow as infiltrative, mineralised masses that extend into and destroy surrounding soft tissue and bone (Bloch et al., 2009). Histologically, chondrosarcomas are divided mainly into conventional and mesenchymal subtypes, with the conventional type being the most common, and mesenchymal accounting for less than 10% of lesions. The mesenchymal type however portends a significantly worse prognosis, with Bloch et al., 2009 demonstrating a 54% 5 year mortality compared to 6% in the conventional type (Bloch et al., 2009).
Other important prognostic factors include the WHO classification of grading, local recurrence and treatment modalities. Evans et al., 1977 reported a 5 year survival for Grades I, II and III of 90%, 81%, and 43% respectively (Evans et al., 1977), and this statistical significance of grading was further confirmed by Bloch et al., 2009 (Bloch et al., 2009).
The US national cancer database indicates a median age of diagnosis of 55, with a male as well as Caucasian predominance (Koch et al., 2000, Bloch and Parsa, 2013). Presentation of these tumours is related to their para-clival location, and specifically to compression of surrounding structures. Most common presenting symptoms include headache and diplopia, with a case series of 33 patients managed by University of California, San Francisco demonstrating over 50% of patients presenting with a 6th cranial nerve palsy (Oghalai et al., 2005, Bloch and Parsa, 2013).
Surgical Management: Expanded Endoscopic Endonasal Approach (EEA)
The current standard of management for intracranial and sinonasal chondrosarcoma is surgical resection with maximal cytoreduction, with a consideration for adjuvant radiotherapy (Bloch and Parsa, 2013, Khan et al., 2013). The aim of surgical resection should be to achieve complete tumour removal, or maximal cytoreduction and decompression of surrounding structures (Gay et al., 1995, Muthukumar et al., 1998, Samii et al., 2009). In a systematic review of case series regarding cranial chondrosarcoma, the 5-year recurrence rate of chondrosarcoma was found to be 44% for patient’s having undergone surgery alone, as compared to 9% in patients undergoing surgery with post-operative radiotherapy (Bloch et al., 2010).
The surgical approach taken for resection of cranial chondrosarcomas is influenced by the location, invasion and size of the actual tumour (Samii et al., 2009). The rarity of these tumours however has inhibited conclusive analysis and consensus on the most effective approach and technique of surgical resection, with data relying largely on small case series. Among tumours with anterior extension into Meckel’s cave, or the cavernous sinus, a fronto-temporal orbito-zygomatic approach has previously been preferred. Laterally, and posterior spreading tumours have been approach via a retrosigmoid approach, or a petrosal approach in the past. In a systematic review, a lateral approach such as retrosigmoid or pterional approach has previously been documented as most common, reflecting the predominance of lateral extension into regions such as the cerebellar-pontine angle, or the posterior cranial fossa (Samii et al., 2009). Endoscopic technological development has led to extended endoscopic endonasal approaches which are now increasingly used for skull base lesions, particularly clival, with excellent surgical outcomes reported in well-selected tumours (Bloch and Parsa, 2013, Frank et al., 2006).
More recently there has been an increase in the use of the Endoscopic endonasal approach in specifically selected chondrosarcomas (Frank et al., 2006, Bloch and Parsa, 2013). Technical reports have outlined the feasibility of using the endoscopic endonasal approach, and more specifically the ‘expanded’ endoscopic endonasal approach for access to skull base tumours such as chondrosarcomas (Bloch and Parsa, 2013, Frank et al., 2006, Kassam et al., 2005a). Although it has been increasingly utilised, as of yet there are limited reports analysing its effectiveness (Frank et al., 2006). Chondrosarcomas have been suggested as ideal tumours for endoscopic endonasal approach given their extradural origin, predominantly extradural growth, the soft tissue of the tumour, and their avascular nature (Gopika Kalsotra, 2010, Frank et al., 2006). Advantages to the endoscopic endonasal approach include the expanded, panoramic field of view, which has been demonstrated by various anatomical studies (Cavallo et al., 2005, Kassam et al., 2005b, Kassam et al., 2005a). It allows visualisation and access to the region between the sellar floor and the foramen magnum in the vertical dimension, and laterally the exposure may be extended to the foramen ovale and rotundum (Figure 5). This improved visualisation can allow a more complete surgical resection as well as greater versatility and access to clival and parasellar masses. Moreover the passage through the nasal cavity is typically safer than other cranial approaches. The expanded endoscopic endonasal approach also prevents the need for brain retraction and hence the associated morbidity of this (Cavallo et al., 2005). It also obviates the need for manipulation of the optic apparatus, minimising the potential for visual dysfunction (Cavallo et al., 2005). Moreover, it allows easier and earlier identification of the pituitary gland and infundibulum increasing the likelihood of preserving neuroendocrine function (Cavallo et al., 2005).
Potential complications associated with the expanded endoscopic endonasal approach to clival masses include the potential for neural and vascular injury, either arterial or venous. Haemostasis has previously been considered challenging, but utilisation of warm irrigation, thrombotic agents and specific endonasal instruments has significantly improved the methods of vascular control. EEA has been reported to have higher rates of CSF leaks compared to other common trans-sphenoidal surgical operations, yet this has been mitigated by the use of reconstructive nasospetal flaps
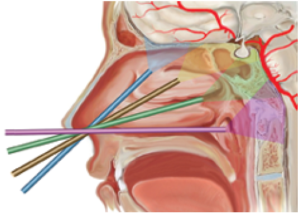
Figure 5: Schema outlining the 4 possible corridors of the midline skull base via an endoscopic endonasal approach. Each trajectory, outlined by a different colour, has a different target. Blue – Olfactory Groove, Yellow – Sella turcica/Planum sphenoidale, Green- Clivus, Purple – Craniovertabral junction/Foramen magnum. Written permission has been obtained for the diagram from Cavallo et al., 2005. (Cavallo et al., 2005)
Reconstructive Options for Endoscopic Skull Base Surgery: Naso-septal flap
Criticisms of the EEA include the high CSF leakage rate. Reported rates of CSF leak with EEA skull-based surgery are around 15.6% when only simple reconstructive measures such as free graft reconstruction are used, as shown by a systematic review (Zanation et al., 2011, Harvey et al., 2009). The introduction of the vascularised free flaps, such as the nasoseptal flap, has reduced this incidence to around 5%, comparable to traditional open techniques (Kassam et al., 2008, Zanation et al., 2011, Harvey et al., 2009).
Before the introduction of vascularised free flaps, reconstructive techniques utilised the use of free tissue grafts. Nasoseptal flaps have generally been considered to provide a more robust repair than free grafts, and anatomical studies have demonstrated that this is a technically feasible option (Harvey, In Press, Shah et al., 2009, Pinheiro-Neto et al., 2007, Harvey et al., 2009). Healing of the intranasal dissection and of the nasoseptal flap is often complete within 6 to 12 weeks (Zanation et al., 2011).
Adjuvant Radiotherapy
The use of adjuvant radiation therapy in combination with surgery is increasingly utilised, reportedly employed in 27.4% of sinonasal chrondrosarcomas diagnosed after 1990 (Khan et al., 2013). Although there is a lack of conclusive statistical evidence given the rarity of these tumours, several small case series have demonstrated some improvement in local recurrence and overall survival (Rosenberg et al., 1999, Oghalai et al., 2005, Bloch and Parsa, 2013). A variety of factors have been shown to influence the decision to employ adjuvant radiation therapy and these include size of tumour being over 5cm, involved neurovascular structures and margin status (Bloch and Parsa, 2013).
There are various radiotherapy modalities available including fractionated photon radiotherapy, proton therapy, particle therapy with ions and stereotactic radiosurgery.
Conventional External Beam Radiation Therapy is the traditional option, delivered via highly conformal techniques such as intensity-modulated radiation therapy. Most analyses show a 5-year progression free survival of around 80% with the use of photon radiotherapy (Bloch and Parsa, 2013). However chondrosarcomas are radio-resistant tumours and doses in excess of 60Gy are required for effective control which can result in radio-toxicity to surrounding structures (Bloch and Parsa, 2013, Amichetti et al., 2010).
More recently the use of Proton therapy has emerged as a viable radiation modality with the advantage of being able to deliver more targeted radiation dosages with fewer toxicity to surrounding structures (Amichetti et al., 2010, Bloch and Parsa, 2013). A recent systematic review of the use of proton therapy in the setting of skull-based chondrosarcomas demonstrated 5-year local control to be between 75-99% (Amichetti et al., 2010).
Other modalities of particle therapy such as carbon ions, and stereotactic radiosurgery are also attractive options that are yet to be conclusively explored (Amichetti et al., 2010).
Given the array of options for adjuvant radiation therapy, the combination with endoscopic approaches is a promising combination. The quicker recovery associated with the EEA is likely to allow an opportunity to move onto early radiotherapy.
Conclusion
The literature regarding surgical management of chondrosarcomas is limited to small case series with a systematic review bringing together only 161 cases (Khan et al., 2013). There is a paucity of consensus regarding the most efficacious surgical approaches to chondrosarcomas located in the clival region. A case of an endoscopic endonasal approach taken to resect a recurrent clival chondrosarcoma with post-operative imaging showing complete resection highlights the success of recent advances in skull base surgery. The adjunct of a naso-septal flap appears to be a successful method of skull base reconstruction. In conclusion, this case endorses a minimally invasive approach to lesions within the posterior clival region as a viable and low morbidity alternative.
References
- Amichetti, M, Amelio, D, Cianchetti, M, Enrici, R. M. and Minniti G. (2010) “A systematic review of proton therapy in the treatment of chondrosarcoma of the skull base,” Neurosurgical Review, 33 (2) 155-65.
- Bloch, O. and Parsa, A.T. (2013) “Skull base chondrosarcoma: evidence-based treatment paradigms,” Neurosurgery Clinics of North America, 24 (1) 89-96.
- Bloch, O. G, Jian, B. J, Yang, I, Han, S. J, Aranda, D, Ahn, B.J. and Parsa, A.T. (2009) “A systematic review of intracranial chondrosarcoma and survival,” Journal of Clinical Neuroscience: official journal of the Neurosurgical Society of Australasia, 16 (12) 1547-51.
- Bloch, O. G, Jian, B. J, Yang, I, han, S.J, Aranda, D, Ahn, B. J. and Parsa, A.T. (2010) “Cranial chondrosarcoma and recurrence,” Skull Base, 20 (3) 149-56.
- Cavallo, L. M, Messina, A, Cappabianca, P, Esposito, F, de Divitiis, E, Gardner, P. and Tschabitscher, M. (2005) “Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations,” Neurosurgical Focus, 19 (1) E2.
- Colli, B. O. and Al-Mefty, O. (2001) “Chordomas of the skull base: follow-up review and prognostic factors,” Neurosurgical Focus, 10 (3)
- Dorfman, H. D. and Czerniak, B. (1995) “Bone cancers,” Cancer, 75 203-10.
- Evans, H. L, Ayala, A. G. and Romsdahl, M. M. (1977) “Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading,” Cancer, 40 (2) 818-31.
- Frank, G, Sciarretta, V, Calbucci, F, Farneti, G, Mazzatenta, D. and Pasquini, E. (2006) “The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas,” Neurosurgery, 59 ONS50-7; discussion ONS50-7.
- Gay, E, Sekhar, L. N, Rubinstein, E, Wright, D. C, Sen, C, Janecka, I. P. and Snyderman, C. H. (1995) “Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients,” Neurosurgery, 36 887-96; discussion 896-7.
- Harvey, P. S. P. A. R. J. In Press. Large Skull Base Defect Reconstruction with and without Pedicled Flaps. El Selvier.
- Harvey, R. J, Nogueira, J. F, Schlosser, R. J, Patel, S. J, Vellutini, E. and Stamm, A. C. (2009) “Closure of large skull base defects after endoscopic transnasal craniotomy. Clinical article,” Journal of Neurosurgery, 111 (2) 371-379.
- Kalsotra, G. and Gupta, A. K. (2010) “Skull Base Chondrosarcoma: Is there a Role of Endoscopic Excision?” Clinical Rhinology: International Scientific Journals from Jaypee, 3 (2) 97-100
- Kassam, A, Snyderman, C. H, Mintz, A, Gardner, P. and Carrau, R. L. (2005a) “Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica,” Neurosurgical Focus, 19 (1) E3.
- Kassam, A, Snyderman, C. H, Mintz, A, Gardner, P. and Carrau, R. L. (2005b) “Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum,” Neurosurgical Focus, 19 (1) E4.
- Kassam, A. B, Thomas, A, Carrau, R. L, Snyderman, C. H, Vescan, A, Prevedello, D, Mintz, A. and Gardner, P. (2008) “Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap,” Neurosurgery, 63, ONS44-52; discussion ONS52-3.
- Khan, M. N, Husain, Q, Kanumuri, V. V, Boghani, Z, Patel, C. R, Liu, J. K. and Eloy, J. A. (2013) “Management of sinonasal chondrosarcoma: a systematic review of 161 patients,” International Forum of Allergy & Rhinology, 3 (8) 670-677.
- Koch, B. B, Karnell, L. H, Hoffman, H.T, Apostolakis, L. W, Robinson, R. A, Zhen, W. and Menck, H. R. (2000) “National cancer database report on chondrosarcoma of the head and neck,” Head & Neck, 22 (4) 408-425.
- Muthukumar, N, Kondziolka, D, Lunsford, L. D. and Flickinger, J. C. (1998) “Stereotactic radiosurgery for chordoma and chondrosarcoma: further experiences,” International Journal of Radiation Oncology, Biology, Physics, 41 (2) 387-392.
- Oghalai, J. S, Buxbaum, J. L, Jackler, R. K. and McDermott, M. W. (2005) “Skull base chondrosarcoma originating from the petroclival junction,” Otology & Neurotology, 26 (5) 1052-1060.
- Pinheiro-Neto, C. D, Prevedello, D. M, Carrau, R. L, Snyderman, C. H, Mintz, A, Gardner, P. and Kassam, A. (2007) “Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study,” The Laryngoscope, 117 (9) 1560-1569.
- Rosenburg, A. E, Nielsen, G. P, Keel, S. B, Renard, L. G, Fitzek, M. M, Munzenrider, J. E. and Liebsch, N. J. (1999) “Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma,” The American Journal of Surgical Pathology, 23 (11) 1370-1378.
- Samaii, A, Gerganov, V, Herold, C, Gharabaghi, A, Hayashi, N. and Samii, M. (2009) “Surgical treatment of skull base chondrosarcomas,” Neurosurgical Review, 32 (1) 67-75; discussion 75.
- Shah, R. N, Surowitz, J. B, Patel, M. R, Huang, B. Y, Synderman, C. H, Carrau, R. L, Kassam, A. B, Germanwala, A. V. and Zanation, A. M. (2009) “Endoscopic pedicled nasoseptal flap reconstruction for pediatric skull base defects,” The Laryngoscope, 119 (6) 1067-1075.
- Zanation, A. M, Thorp, B. D, Parmar, P. and Harvey, R. J. (2011) “Reconstructive options for endoscopic skull base surgery,” Otolaryngologic Clinics of North America, 44 (5) 1201-1222.







