There is evidence of a strong association between childhood urinary tract infection, vesicoureteral reflux and the development of renal scars with consecutive loss of kidney function. The predisposing pathophysiological conditions that interfere with different clinical features in the individual child over time, however, are a widely discussed representing a complicated issue that renders management of vesicoureteral reflux challenging as discussed in publications by Koyle 2011 and Coulthard 2008. Age, sex, reflux maturation and the concept of outgrowing the risk of scarring as well as decreasing risk for febrile urinary tract infections have always been important points in stratifying the risk for new scar formation and indication for therapy. It was widely acknowledged that older children or adults are at lower risk for new scar formation. This view is being challenged by growing evidence that vesicoureteral reflux and infection have an impact on kidney function in adults or transplanted patients as shown in a study by Coulthard et al. (2006). Additionally great importance was attached to the classic concepts of Ransley and Risdon 1975 who demonstrated that scarring is depending on intrarenal reflux which occurs in defined anatomic locations at the kidney poles that are present in about 70% of human beings.
Case Report
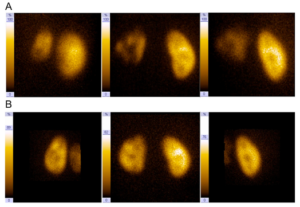
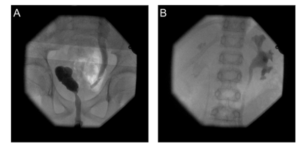
MJ was referred to our department after a first febrile urinary tract infection (Enterococcus faecalis) in his first month of life. Initially, bilateral high-grade (°III-IV) reflux with an inconspicuous urethra was diagnosed on VCUG. Consequently the boy got prophylactic antibiosis and a circumcision to lower the risk for further UTIs was performed. MAG3 nephrography performed at this time because of bilateral hydronephrosis and bilaterally dilated ureters showed a split function of 49% on the left and 51% on the right side. Sonographically both kidneys appeared normal. Serum creatinin at this time was 0,43mg/dl, corresponding to an eGFR of 84,76ml/min (Schwartz formula, body height 81cm). He had no other health problems and there was no history of VRR or other diseases of the kidneys or urogenital tracts in his family. At the age of 1 year, with no breakthrough infections in the meantime, VCUG was repeated and demonstrated grade II reflux on both sides. A DMSA scan demonstrated postpyelonephritic scarring on both sides with a split renal function of 41% on the left and 59% on the right side. Consequently, bilateral endoscopic therapy with injection of dextranomer/hyaluronic acid copolymer (Deflux®) was performed. The endoscopic evaluation of the urethra appeared normal without any sign of posterior urethral valves. In the control VCUG three months after therapy a persistent reflux grade II on the right side was verified. Consequently, four months after initial endoscopic therapy, at the age of 17 months, an open unilateral extravesical anti-reflux surgery (Lich Gregoir) was performed. In the follow up, for 10 years, no urinary tract infections occurred. There was no clinical sign of lower urinary tract dysfunction or bowel dysfunction. As well there were no other infections or signs of immunodeficiency. Sonographically the left kidney appeared smaller but demonstrated no hydronephrosis. A DMSA scan performed four years after therapy (Fig/ 1B)
demonstrated persistent bilateral grade II reflux. In order to exclude bladder dysfunction a videourodynamic study was performed, filling and micturition pressures were normal; no sphincter-detrusor dyscoordination could be demonstrated, no residual urine was demonstrable. Magnetic resonance tomography of the spine showed no pathological changes, either. At the age of 11 years and 6 months, bilateral ureteral reimplantation (Cohen technique) was performed. Up to now (12 months) there was no further UTI and no clinical sign of bladder or bowel dysfunction as well as no hydronephrosis or other pathological changes on sonography.
Discussion
There is consensus on the fact that the development of renal scarring requires vesicoureteral — and probably intrarenal — reflux as well as urinary tract infection simultaneously. On the other hand dysplastic renal changes, often associated to VRR and probably with a common embryologic background, are static and present since birth without change during further life. Older children are generally thought to be at considerably lower risk for new renal scar formation. One the one hand this may be due to a lesser risk of kidney scarring with older age. This concept of kidneys becoming resistant to new damage with age is challenged by numerous studies e.g. it has been proven in pigs as well as in transplanted adult kidneys that reflux together with urinary tract infections induces renal scars as detailed above. On the other hand, the lower risk of scarring in older patients may be mainly due to the maturation of reflux or respectively its successful treatment. Maturation of reflux is, as well as its origination not well understood and may depend on the one hand on anatomical changes of the vesicoureteral junction as shown by Oswald et al. as well as on changes in intravesical pressure profile as illustrated by the results of the Swedish reflux Study (Silén et al. 2010). Our patient has mainly acquired loss of kidney function due to recurrent UTIs with no or no significant underlying dysplasia as demonstrated by the initial equal split renal function.
Lower urinary tract as well as bowel dysfunction is a known risk factor of persistent reflux and probably a sign of incomplete or dysfunctional bladder maturation. The same risk factors may be relevant for avoiding recurrence of VUR after surgical interventions. While reflux recurrence after open reflux surgery is reported to be rare, recurrent reflux after endoscopic therapy is a known phenomenon with a wide range of possible incidence in literature. Our patient, however, recurred twice after endoscopic and open reflux surgery without having LUTD or bowel dysfunction.
It has been shown by Coulthard et al. 2006 that scarring with consecutive loss of function may occur during one single febrile urinary tract infection even if it is treated properly within 48h. This may explain the marked loss of kidney function during only one episode of UTI in our patient.
Why our patient developed UTIs, however, is difficult to explain in a circumcised boy with absence of bladder or bowel dysfunction and a normal urethra despite the bilateral reflux. Our patient certainly belongs to a risk group for developing new infections, having had an abnormal DMSA scan beforehand, as Mingin et al. (2004) show in their evaluation of 120 patients. There is data for example by Chiou et al. (2001) to support a greater risk of scarring in older patients due to a late diagnosis and longer courses of UTI as happened in our patient. No or minor clinical signs do not predict lower rates of scarring and are more often seen in older children in comparison to younger ones, as Coulthard et al. (2009) have shown. One factor that can’t be omitted is a possible role of the surgery in generating a bladder dysfunction. We were not able to proof a bladder emptying problem in our examinations, though it can’t be excluded that after unilateral extravesical ureteral reimplantation bladder emptying could be harmed to an extent facilitating the onset of a UTI.
Conclusion
Other than it is commonly believed kidneys do not outgrow the risk of scarring and loss of function due to VRR and UTIs. Although the risk in older children seems to be considerably lower, a certain population of patients having no bladder or bowel dysfunction are still at risk for late recurrence of VRR and consecutive harm to kidney function. Our case report illustrates the importance of long term follow up and consequent diagnosis and treatment for patients after successful therapy of VRR in early childhood.
References
1. Chiou Y. Y., Wang S. T., and Tang M. J. (2001) “Renal fibrosis: prediction from acute pyelonephritis focus volume measured at 99mTc dimercaptosuccinic acid SPECT”. Radiology 221, 366-70.
Publisher – Google Scholar
2. Coulthard M. G., Lambert H. J. and Keir M. (2009) “Do systemic symptoms predict the risk of kidney scarring after urinary tract infection?” J.Arch Dis Child, 94(4), 278-81.
Publisher – Google Scholar
3. Coulthard M. G., Flecknell P. and Orr H. (2002) “Renal scarring caused by vesicoureteric reflux and urinary infection: a study in pigs.” Pediatr Nephrol, (17), 481-4.
Google Scholar
4. Coulthard M.G. and Keir M.J. (2006) “Reflux nephropathy in kidney transplants, demonstrated by dimercaptosuccinic acid scanning”. Transplantation, 82, 205-10.
Publisher – Google Scholar
5. Coulthard M.G. (2008) “Is reflux nephropathy preventable, and will the NICE childhood UTI guidelines help?”. Arch Dis Child, 93, 196-9.
Publisher – Google Scholar
6. Holmdahl, G, Brandström, P, and Läckgren, G, (2010) „The Swedish reflux trial in children: II. Vesicoureteral reflux outcome“. J Urol, 184, 280-285.
Publisher – Google Scholar
7. Koyle M.A., ElderJ.S., and Skoog S.J. (2011) “Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation”. Pediatr Surg Int, 27, 337-46.
Publisher – Google Scholar
8. Mingin, G.C., Nguyen H.T. and Baskin L.S. (2004) “Abnormal Dimercapto-succinic Acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux.” The Journal of Urology 172, 1075-1077.
Publisher – Google Scholar
9. Oswald J, Schwentner C., and Brenner E. (2004) „Extracellular matrix degradation and reduced nerve supply in refluxing ureteral endings”. J Urol 172, 1099-1102.
Publisher – Google Scholar
10. Oswald J., Schwentner C., and Lunacek A. (2006) „Reevaluation of the fetal muscle development of the vesical trigone.” J Urol 176, 1166-1170.
Publisher – Google Scholar
11. Ransley P.G. and Risdon R.A. (1975) “Renal papillary morphology and intrarenal reflux in the young pig.” Urol Res, 3, 105-9.
Publisher – Google Scholar
12. Ransley P.G., Risdon R.A. (1975) “Renal papillary morphology in infants and young children.” Urol Res, 3, 111-3.
Publisher – Google Scholar
13. Sillén U., Brandström P. and Jodal U. (2010) “The Swedish reflux trial in children: V. Bladder dysfunction.” J Urol, 184, 298-304.
Publisher – Google Scholar