Introduction
Simultaneous bilateral ICHs are exceptionally rare and an association with carbon monoxide poisoning is yet to be described (Neau et al., 1997). Only 19 case reports exist to date that describe an association between carbon monoxide poisoning and haemorrhagic infarction. Whilst the chronic effects of carbon monoxide poisoning are poorly understood, we propose that in this case carbon monoxide poisoning may have been a significant predisposing factor to the development of basal ganglia haemorrhage. To our knowledge, there have been no reported cases of carbon monoxide poisoning leading to ICH.
Moreover, the utilisation of decompressive craniectomy in the management of simultaneous bilateral ICH has not previously been reported. The rational of the operative procedure in the setting of ICH is to prevent the associated haematoma mass effect as well as the toxic effects of haematoma degradation (Fung et al., 2012). Decompressive craniectomy has been successfully applied in various other causes of intracranial space-occupying pathology, including major ischaemic stroke and trauma, with studies showing significant clinical benefit. The role of decompressive craniectomy in the setting of ICH, however, is far more questionable, and current studies remain inconclusive about its value (Fung et al., 2012). However there is evidence of benefit in certain subgroups of patients with ICH, and we maintain that its role in the setting of ICH has not yet been fully elucidated.
Case Report
A 49 year old Afro-Caribbean man was found unconscious at home with vomit around their mouth, and evidence of both faecal and urinary incontinence. The patient had no significant past medical history, lived alone, and worked as a data analyst. During their hospital stay it emerged that one month prior to admission, the patient had been fixing his boiler, when he involuntarily inhaled a large volume of released gas, carbon monoxide (CO).
On admission, the patient was tachycardic (130bpm) and hypertensive (160/90mmHg) with a Glasgow Coma Scale (GCS) of 5 (E1V1M3). He was resuscitated, intubated and ventilated, and a CT head performed. This revealed bilateral basal ganglia haemorrhages (Figure 1).
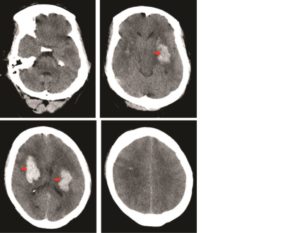
Figure 1: Axial CT head showing bilateral basal ganglia haemorrhages (red arrows). The sulci, ventricles, and cisterns are effaced demonstrating the significant mass effect exerted by the haemorrhages
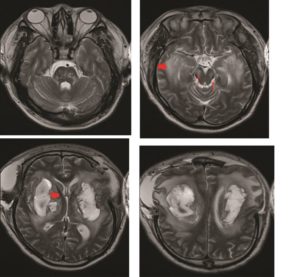
The raised intracranial pressure (ICP) was refractory to medical management, and the patient underwent urgent bilateral frontotemporal decompressive craniectomies with durotomies. Post-operative continuous ICP monitoring confirmed values within normal limits and imaging revealed satisfactory decompression (Figure 2). The patient was managed in the neurosurgical intensive therapy unit (ITU) for four weeks, during which time a surgical tracheostomy was performed. They did not succumb to their disease, but improved neurologically, with preserved brainstem reflexes. Subsequent MRI of the brain revealed additional foci of haemorrhage within the pons (Figure 3), however, an MR-Angiogram did not reveal any abnormal intracranial vessels.
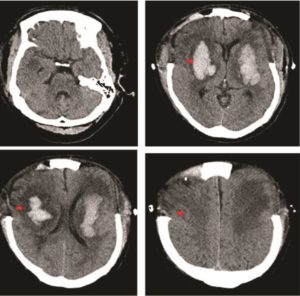
Figure 2: Axial CT head at day 4 post operatively illustrating bifrontal decompressive craniectomies and resolving basal ganglia haemorrhages (red arrows)
Figure 3: Axial T2 weighted MRI head undertaken at day 14 post operatively again showing the resolving bilateral basal ganglia haemorrhages (thick red arrows), with perilesional oedema, and the haemorrhagic foci within the pons (small red arrows).
The patient was transferred to their local ITU with a GCS of 6 (E2, V1, M3). At discharge from our unit, the patient had been successfully weaned off the ventilator, and was breathing independently. His brain stem reflexes were all intact, however he remained quadriplegic.
Discussion
Simultaneous bilateral basal ganglia haemorrhages are extremely rare (Neau et al., 1997). Whilst hypertension remains a dominant identifiable risk factor in these patients, varied causes have been reported including cerebral amyloid angiopathy (Tucker, Bilbao, & Klodawsky, 1980), vasculitis (Verma, 2006), and neoplasms (Hickey, King, Wang, & Samuels, 1983).
Bilateral ICH typically affects the thalamic and putaminal regions, not unlike unilateral solitary ICH (Neau et al., 1997). However, the former have been shown to carry a grave prognosis. This is thought to be linked to the concomitant effect on both decussating and non-decussating tracts. The uncrossed pyramidal tracts and the sensory tracts may remain unaffected in a solitary, unilateral ICH and hence aid neurological recovery. However in bilateral ICH, these tracts are compromised, dramatically worsening neurological dysfunction, and therefore impacting negatively on morbidity and mortality. Furthermore, the diaschisis effect of bilateral ICH exaggerates the neurological deficits of these patients.
Carbon monoxide as a cause of intracerebral haemorrhage
In the case described, the CO leak from the boiler was the only identifiable risk factor. CO poisoning is known to have many neurological sequela, including diffuse hypoxic-ischaemic encephalopathy (Bianco & Floris, 1996), necrosis of the globus pallidus (Prockop & Chichkova, 2007), and “spongy change in the cerebral cortex” (El Khashab & Nejat, 2009). Although a rarer occurrence, CO toxicity presenting as cerebral haemorrhagic infarction has been reported (Bianco & Floris, 1996; El Khashab & Nejat, 2009).
To our knowledge, there are no reported cases of ICH occurring as a late effect of CO exposure. The history is supported by the appearance of multiple foci of haemorrhage and necrosis, consistent with MRI images previously described in CO poisoning.(Bianco & Floris, 1996) CO toxicity has two predominant mechanisms of injury, namely, hypoxic and a metabolic cellular injury. CO has at least 200 times the affinity for hemoglobin than oxygen; hence CO is able to competitively inhibit oxygen transport by restricting the function of haemoglobin. This results in hypoxic injury (Ernst & Zibrak, 1998). With regard to metabolic cellular injury, this is related to mitochondrial dysfunction and the activation of polymorphonuclear leukocytes with brain lipid peroxidation (Lo et al., 2007). These mechanisms lead to central nervous system complications via many pathways, and more complex downstream metabolic changes. One of the major mechanisms is through injury to the endothelium and vascular compromise within the brain (Quinn et al., 2009). This is supported by neuroimaging evidence of foci of haemorrhage and necrosis occurring after CO toxicity (Bianco & Floris, 1996), as well as haemorrhagic infarcts occurring as a complication of CO exposure (Bianco & Floris, 1996; Finelli & DiMario, 2004). This is highly significant with regards to a link to ICH development, as small vessel damage is integral to the pathogenesis of ICH (Smith & Eskey, 2011). The major recognised causes of ICH, including hypertension and cerebral amyloid angiopathy, lead to haemorrhage via gradual damage and weakening of the small vessels of the brain (Smith & Eskey, 2011; Thanvi & Robinson, 2006). Therefore, the evidence of CO damage to the penetrating vessels of the brain describes a rational predisposition to the development of ICH.
Bilateral Decompressive Craniectomy
Decompressive craniectomy is a controversial method of managing increased intracranial pressure, most commonly performed in the setting of major ischaemic stroke, traumatic brain injury, and subarachnoid haemorrhage due to vascular abnormality, however there is far less literature regarding its use in the management of ICH (Fung et al., 2012). Decompressive craniectomy has been proven to reduce mortality significantly in the setting of ischaemic infarction, most dramatically via the results of the Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery II (DESTINY II) study (Juttler et al., 2014). The trial demonstrated that early decompressive hemicraniectomy for patients aged 61 years or older with extensive MCA infarction resulted in an increase in the proportion of patients surviving without severe disability from 18% in the control group, to 38% in the hemicraniectomy group (p=0.04) (Juttler et al., 2014). Moreover, in the setting of traumatic brain injury it is a proven method of controlling intracranial pressure rise, however the current evidence does not yet suggest that it confers a survival advantage (Sahuquillo, Martinez-Ricarte, & Poca, 2013). The role of decompressive craniectomy in the management of ICH, however, is far more controversial with a large prospective trial demonstrating no mortality benefit (Mendelow et al., 2005). However a subsequent trial that was performed, the STICH II trial (Mendelow et al., 2013), did demonstrate clinical benefit in certain subgroups such as superficial ICH without intra-ventricular extension. To our knowledge, however, there are no reports of the effectiveness of bilateral decompressive craniectomies in the management of bilateral spontaneous ICH. However, our case demonstrates that when performed urgently, it may be effective in reducing medically intractable raised intracranial pressure and preserve life.
Conclusion
We report a rare case of simultaneous bilateral ICH secondary to CO exposure managed with bilateral decompressive craniectomies. The report highlights an association with preceding carbon monoxide poisoning, and proposes a causative association that has not been reported previously in the literature. Whilst no causative association has been proven in this case, we have outlined a rational pathway in which CO poisoning may predispose to the development of bilateral ICH.
References
1. Bianco, F., & Floris, R. (1996). MRI appearances consistent with haemorrhagic infarction as an early manifestation of carbon monoxide poisoning. Neuroradiology, 38 Suppl 1, S70-72.
Publisher – GoogleScholar
2. El Khashab, M., & Nejat, F. (2009). Hemorrhagic cerebral infarction in carbon monoxide poisoning: a case report.Cases J, 2, 96. doi: 10.1186/1757-1626-2-96
Publisher – GoogleScholar
3. Ernst, A., & Zibrak, J. D. (1998). Carbon monoxide poisoning. N Engl J Med, 339(22), 1603-1608. doi: 10.1056/NEJM199811263392206
Publisher – GoogleScholar
4. Finelli, P. F., & DiMario, F. J., Jr. (2004). Hemorrhagic infarction in white matter following acute carbon monoxide poisoning. Neurology, 63(6), 1102-1104.
Publisher – GoogleScholar
5. Fung, C., Murek, M., Z’Graggen, W. J., Krahenbuhl, A. K., Gautschi, O. P., Schucht, P., . . . Beck, J. (2012).Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke, 43(12), 3207-3211. doi: 10.1161/STROKEAHA.112.666537
Publisher – GoogleScholar
6. Hickey, W. F., King, R. B., Wang, A. M., & Samuels, M. A. (1983). Multiple simultaneous intracerebral hematomas. Clinical, radiologic, and pathologic findings in two patients. Arch Neurol, 40(8), 519-522.
Publisher – GoogleScholar
7. Juttler, E., Unterberg, A., Woitzik, J., Bosel, J., Amiri, H., Sakowitz, O. W., . . . Investigators, D. I. (2014).Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med, 370(12), 1091-1100. doi: 10.1056/NEJMoa1311367
Publisher – GoogleScholar
8. Lo, C. P., Chen, S. Y., Lee, K. W., Chen, W. L., Chen, C. Y., Hsueh, C. J., & Huang, G. S. (2007). Brain injury after acute carbon monoxide poisoning: early and late complications. AJR Am J Roentgenol, 189(4), W205-211. doi: 10.2214/AJR.07.2425
Publisher – GoogleScholar
9. Mendelow, A. D., Gregson, B. A., Fernandes, H. M., Murray, G. D., Teasdale, G. M., Hope, D. T., . . . investigators, S. (2005). Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet, 365(9457), 387-397. doi: 10.1016/S0140-6736(05)17826-X
Publisher – GoogleScholar
10. Mendelow, A. D., Gregson, B. A., Rowan, E. N., Murray, G. D., Gholkar, A., Mitchell, P. M., & Investigators, S. I. (2013). Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet, 382(9890), 397-408. doi: 10.1016/S0140-6736(13)60986-1
Publisher – GoogleScholar
11. Neau, J. P., Ingrand, P., Couderq, C., Rosier, M. P., Bailbe, M., Dumas, P., . . . Gil, R. (1997). Recurrent intracerebral hemorrhage. Neurology, 49(1), 106-113.
Publisher – GoogleScholar
12. Prockop, L. D., & Chichkova, R. I. (2007). Carbon monoxide intoxication: an updated review. J Neurol Sci, 262(1-2), 122-130. doi: 10.1016/j.jns.2007.06.037
Publisher – GoogleScholar
13. Quinn, D. K., McGahee, S. M., Politte, L. C., Duncan, G. N., Cusin, C., Hopwood, C. J., & Stern, T. A. (2009).Complications of carbon monoxide poisoning: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry, 11(2), 74-79.
Publisher – GoogleScholar
14. Sahuquillo, J., Martinez-Ricarte, F., & Poca, M. A. (2013). Decompressive craniectomy in traumatic brain injury after the DECRA trial. Where do we stand? Curr Opin Crit Care, 19(2), 101-106. doi: 10.1097/MCC.0b013e32835eba1a
Publisher – GoogleScholar
15. Smith, S. D., & Eskey, C. J. (2011). Hemorrhagic stroke. Radiol Clin North Am, 49(1), 27-45. doi: 10.1016/j.rcl.2010.07.011
Publisher – GoogleScholar
16. Thanvi, B., & Robinson, T. (2006). Sporadic cerebral amyloid angiopathy–an important cause of cerebral haemorrhage in older people. Age Ageing, 35(6), 565-571. doi: 10.1093/ageing/afl108
Publisher – GoogleScholar
17. Tucker, W. S., Bilbao, J. M., & Klodawsky, H. (1980). Cerebral amyloid angiopathy and multiple intracerebral hematomas. Neurosurgery, 7(6), 611-614.
Publisher – GoogleScholar
18. Verma, A. (2006). Bilateral basal ganglionic hemorrhage. Arch Neurol, 63(3), 464. doi: 10.1001/archneur.63.3.464
Publisher – GoogleScholar