Discussion
In our case, for the first time in Colombia, an Epitheliotrophic Canine Cutaneous T-cell Lymphoma (CETL) is reported. CETL is a rare neoplasia of unknown etiology (Fournel-Fleury et al., 2002; Schmidt, 2011). The disease is characterized by a neoplastic T-cell infiltration with a special tropism toward the epidermis and related structures. The disease has 3 clinical forms: mycosis fungoides (MF), pagetoid reticulosis (PR) and Sezary syndrome (SS). Although lymphoma is a common neoplasia in dogs, this is not the case in cutaneous form, which represents only 5% of the overall canine lymphomas (Fournel-Fleury et al., 2002; Schmidt, 2011). Lymphoma occurs in adult dogs, older than 9 years, with no predilection for breed or gender (Fontaine et al., 2009; Scott et al., 2001). However, because of the most frequently detection in Boxer and Cocker spaniel, several authors have proposed that it depends on the predominant breeds in each country (Fontaine et al., 2009). Curiously, the present case, the patient was a 7 years old Golden retriever. The etiology of the disease is not clear, although abnormal Langerhans cell function (Weinstock and Horm, 1988) and the type C feline leukemia virus have been associated to the SS form in dogs (Safran et al., 1992). No association to the previous chronic dermatitis of the patient with CETL has been found (Shapiro and Pinto, 1994), but an association between CETL and other pathologies, such as previous atopic dermatitis, has been suggested (Santoro, 2007).
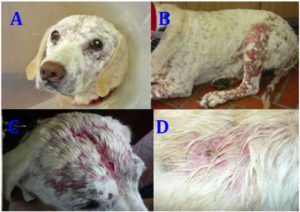
Clinical signs of CETL include single or multiple plaques of variable size from a few millimeters to several centimeters of diameter. Depigmentation of the mucocutaneous limits is also present, as well as ulcers in skin and mouth, where a severe stomatitis with generalized erythema, alopecia, intense pruritus and scratching has also been found. In some dogs, the digital pulps become hyper keratotic. When the disease progresses to chronicity, peripheral lymphadenomegaly and systemic disease are observed (Bhang et al., 2006; Hnilica and Medleau, 2011; Schmidt, 2011; Scott et al., 2001). This case presented clinical signs similar to those described by Foster et al (1997) (Foster et al., 1997), such as plaques, mucocutaneous lesions, severe pruritus and scratching, exfoliative erythrodermia, alopecia and wet skin with sero-purulent secretion (Figure 1). In addition, the present case exhibited clinical signs of systemic disease, bronco-pneumonia and fever for the last month of life.
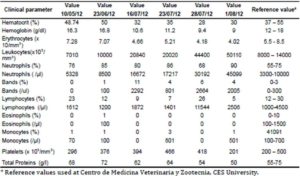
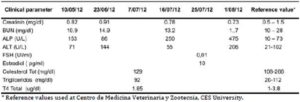
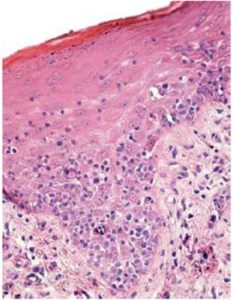
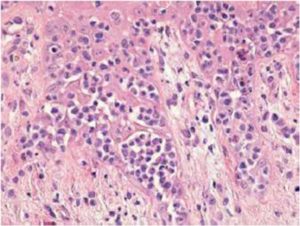
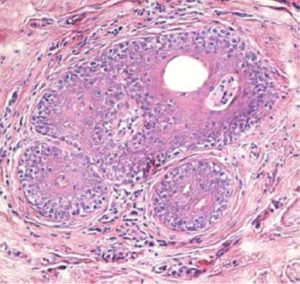
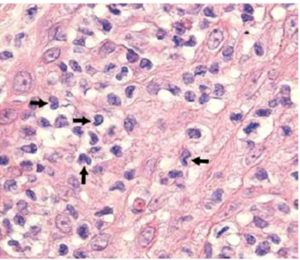
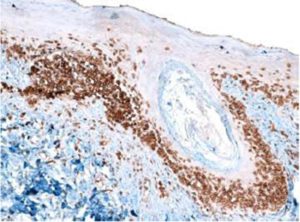
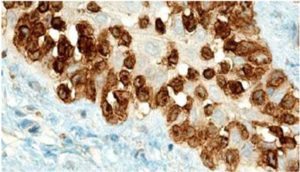
Sezary syndrome is characterized by dermatological clinical signs accompanied with lymphocytic and neutrophilic leukocytosis (59.000/mm3) (Ghernati et al., 1999). This case showed several dermatological signs that were further confirmed by histopathology, including fungoid mycoses and a progressive increase of leukocyte counts until neutrophilia with left deviation, anemia and hypoproteinemia, but normal platelet count. Creatinine and BUN values were always found within the normal range, whereas alkaline phosphatase (ALP) and ALT values proportionally increased with skin and general compromise (Tables 1 and 2). Microscopic findings consist in lympho-proliferative lesions in epidermis with variable collections of atypic lymphocytes (Pautrier’s micro abscesses). Contrary to human mycosis fungoides, in dogs lesions the lymphocytes are CD8+ and frequently exhibit a severe compromise of eccrine and pilous annexes with no associated mucinosis. Interestingly, there were not any intra-cytoplasmic granules or clear cytoplasm in T cells, so excluding a granular or monocytoid T-cell lymphoma. Frequently, the nucleus is small, without nucleolus and angled (Fontaine et al., 2009). As above-mentioned, the skin lesion of this case predominantly showed CD3+ T-cells, but also as mentioned, in peripheral blood smears performed during medical evaluations no Sezary`s cells were observed, suggesting Sezary syndrome. Monoclonal antibodies directed against human CD8 T-cells were also tested, but no signal was detected.
Differential Diagnosis
If the patient presents exfoliative erythrodermia, the differential diagnosis may include canine sarcoptic mange, atopic dermatitis, food adverse reactions, vasculitis, alteration of keratinization and Leishmaniasis, and in case of individual or multi-nodular plaques, differential diagnosis may include skin neoplasia, infection and hystiocytosis. If mucocutaneous lesions are detected, the diagnosis may include lupus erythematous, mucocutaneous pyoderm, vulgar pemphigus, paraneoplasic pemphigus, sub-epidermal paraneoplasic diseases, erythema multiform and complex vasculitis. Finally, if the dog exhibits oral ulcers, differential diagnosis may include autoimmune pemphigus and chronic stomatitis (Schmidt, 2011). In the present case, an acute pyoderm was considered in the initial differential diagnosis based on findings of a skin cytology compatible with Staphylococcus psuedointermedius and Malassezia pachidermatis infection, and as a result of the disseminated sero-purulent secretion found in the skin. The smear did not reveal the presence of mites. In addition, hormonal tests gave no related results (In fact, the patient was sprayed for avoiding the potential deleterious effect of estrogens in the skin) (Table 2). When a definitive diagnosis was reached, the patient was treated with several medications that may cause a significant toxicity in the skin and in different organs. Therefore, you must have knowledge in Internal Medicine and Dermatology diagnoses to establish a rapid and therefore adequate treatment for an established patient prognosis and survival thereof.
The prognosis for patients suffering CETL is poor and it is important to define subtypes present in an affected dog in order to established survival times. The mean survival time vary from 2 months to 2 years (Scott et al., 2001). In early diagnosis of dogs presenting patches or plaques, survival time ranges from 8 to 12 months (de Lorimier, 2006), although an average survival time up to 6 months after a definite diagnosis was proposed (Fontaine et al., 2010). This case was attended after a 4 month period of unsuccessful treatment elsewhere and the patient died 4 month after our treatment schedules had begun.
Treatment of CETL must be focused on palliative management of pain and pruritus, although some patients did not respond to this therapy. In fact, some practitioners did consider euthanasia as a choice because of humanitarian reasons (Schmidt, 2011). The myelosuppressive agent lomustine (CCNU) has been used for treatment of CETL, resulting in a 106 days average remission time in 78% of patients, and 16% overall remission in the affected dogs (Williams et al., 2006). Other study reported 83% overall remission with a 94 days average remission time (Risbon et al., 2006). The use of retinoids during two months has resulted in partial remission of signs in 50 % of patients when combined with prednisone for the treatment of pruritus and topic chemotherapy (Ettinger, 2003). In the present case did not respond to antibiotics, analgesia and topic shampooing, and when treated with triamcinolone, the patient presented a remission only for a short period of time (3 weeks), followed by a severe relapse of signs.
Surgical excision of unique lesions must be accompanied with chemotherapy or topic treatment with glucocorticoids (GC), mecloretamin, carmustin, retinoids, and imiquimod (de Lorimier, 2006). Other chemotherapeutic agents used in addition to lomustine are GC, mainly as coadjuvants; L-asparaginase, dacarbazin and doxorubicin with no concluding results. When a combined chemotherapy is used –such as cyclophosphamide, doxorubicin, vincristine, and prednisone–, the remission rates range from 30% to 50% (de Lorimier, 2006). When a definite diagnosis of mycosis fungoides was established in this case, the patient presented a severe episode of bronchopneumonia before starting chemotherapy, and died 15 days later.
Conclusions
There is a wide range of clinical signs in CETL, including from the distinctive skin lesions to relevant hematological disorders not observed in other skin diseases which can be established as differential diagnosis. In Colombia, this neoplasia is not identified, either by a little knowledge, lack of reporting or because it is not really a common pathology, so it is therefore important to state that the above-mentioned neoplasia can be observed in the routine veterinarian visit and that there is a need to have diagnostic criteria available allowing clinicians to get ready a presumptive diagnosis and that the appropriate diagnostic measures are taken in order to establish quick diagnosis, and therefore, predictions and possible treatments for giving quality of life to the patients affected by this pathology
Acknowledgements
INCA-CES research group activities are funded by CES University (Medellin, Colombia). Authors thank Dr. Juan Maldonado-Estrada (University of Antioquia) for critical reviewing of the manuscript.
References
Ackerman, L. J. (2008). “Atlas de Dermatología en Pequeños Animales,” Inter-Médica, Buenos Aires.
Publisher – Google Scholar
Bhang, D. H., Choi, U. S., Kim, M. K., Choi, E.- H., Kang, M.- S., Hwang, C.-Y., Kim, D.- Y., Youn, H. Y. & Lee, C.W. (2006). “Epitheliotropic Cutaneous Lymphoma (Mycosis Fungoides) in a Dog,” Journal of Veterinary Science 7, 97—99.
Publisher – Google Scholar
De Lorimier, L.- P. (2006). “Updates on the Management of Canine Epitheliotropic Cutaneous T-Cell Lymphoma,”Veterinary Clinics of North America: Small Animal Practice 36, 213—228.
Publisher – Google Scholar
Ettinger, S. N. (2003). “Principles of Treatment for Canine Lymphoma,” Clinical Techniques in Small Animal Practice 18, 92—97.
Publisher – Google Scholar
Fogel, Y. M. & Manzuc, P. (2009). ‘Dermatología Canina para la Práctica Clínica Diaria,’ Intermedica, Buenos Aires.
Google Scholar
Fontaine, J., Bovens, C., Bettenay, S. & Mueller, R. S. (2009). “Canine Cutaneous Epitheliotropic T-Cell Lymphoma: A Review,” Veterinary and Comparative Oncology 7, 1—14.
Publisher – Google Scholar
Fontaine, J., Heimann, M. & Day, M. J. (2010). “Canine Cutaneous Epitheliotropic T-Cell Lymphoma: A Review of 30 Cases,” Veterinary Dermatology 21, 267—275.
Publisher – Google Scholar
Foster, A. P., Evans, E., Kerlin, R. L. & Vail, D. M. (1997). “Cutaneous T-Cell Lymphoma with Sézary Syndrome in a Dog,” Veterinary Clinical Pathology 26, 188—192.
Publisher – Google Scholar
Fournel-Fleury, C., Ponce, F., Felman, P., Blavier, A., Bonnefont, C., Chabanne, L., Marchal, T., Cadore, J. L., Goy-Thollot, I., Ledieu, D., Ghernati, I. & Magnol, J. P. (2002). “Canine T-Cell Lymphomas: A Morphological, Immunological, and Clinical Study of 46 New Cases,” Veterinary Pathology 39, 92—109.
Publisher – Google Scholar
Ghernati, I., Auger, C., Chabanne, L., Corbin, A., Bonnefont, C., Magnol, J. P., Fournel, C., Rivoire, A. & Monier, J. C., Rigal, D. (1999). “Characterization of a Canine Long-Term T Cell Line (DLC 01) Established from a Dog with Sézary Syndrome and Producing Retroviral Particles,” Leukemia 13, 1281—1290.
Publisher – Google Scholar
Harvey, R. G. & McKeever, P. J. (2001). ‘Manual Ilustrado de Enfermedades de la Piel en Perro y Gato,’ EDIMSA, Madrid.
Google Scholar
Hnilica, K. A. & Medleau, L. (2011). ‘Small Animal Dermatology”¯: A Color Atlas and Therapeutic Guide,’Elsevier/Saunders, St. Louis, Mo.
Medleau, L., Hnilica, K. A., Fagliari, J. J. & Fagliari, G. S. (2003). ‘Dermatologia de Pequenos Animais: Atlas Colorido e Guia Terapêutico,’ Roca, São Paulo.
Google Scholar
Risbon, R. E., de Lorimier, L. P., Skorupski, K., Burgess, K. E., Bergman, P. J., Carreras, J., Hahn, K., Leblanc, A., Turek, M., Impellizeri, J., Fred, R., 3rd, Wojcieszyn, J. W., Drobatz, K. & Clifford, C. A. (2006). “Response of Canine Cutaneous Epitheliotropic Lymphoma to Lomustine (CCNU): A Retrospective Study of 46 Cases (1999-2004),” Journal of Veterinary Internal Medicine 20, 1389—1397.
Publisher – Google Scholar
Safran, N., Perk, K. & Eyal, O. (1992). “Isolation and Preliminary Characterisation of a Novel Retrovirus Isolated from a Leukaemic Dog,” Research in Veterinary Science 42, 250—255.
Publisher – Google Scholar
Schmidt, V. (2011). “Epitheliotropic T-Cell Cutaneous Lymphoma in Dogs,” Companion Animal 16, 49—54.
Publisher – Google Scholar
Scott, D. W., Muller, G. H., Kirk, R. W., Miller, W. H. & Griffin, C. E. (2001). ‘Muller & Kirk’s Small Animal Dermatology,’W.B. Saunders, Philadelphia.
Google Scholar
Shapiro, P. E. & Pinto, F. J. (1994). “The Histologic Spectrum of Mycosis Fungoides/Sézary Syndrome (Cutaneous T-Cell Lymphoma). A Review of 222 Biopsies, Including Newly Described Patterns and the Earliest Pathologic Changes,”American Journal of Surgical Pathology 18, 645—667.
Publisher – Google Scholar
Weinstock, M. A. & Horm, J. W. (1988). “Mycosis Fungoides in the United States Increasing Incidence and Descriptive Epidemiology,” JAMA, Journal american medical asociation 260, 4—46.
Publisher – Google Scholar
Williams, L. E., Rassnick, K. M., Power, H. T., Lana, S. E., Morrison-Collister, K. E., Hansen, K. & Johnson, J. L. (2006). “CCNU in the Treatment of Canine Epitheliotropic Lymphoma,” Journal of Veterinary Internal Medicine 20, 136—143.
Publisher – Google Scholar