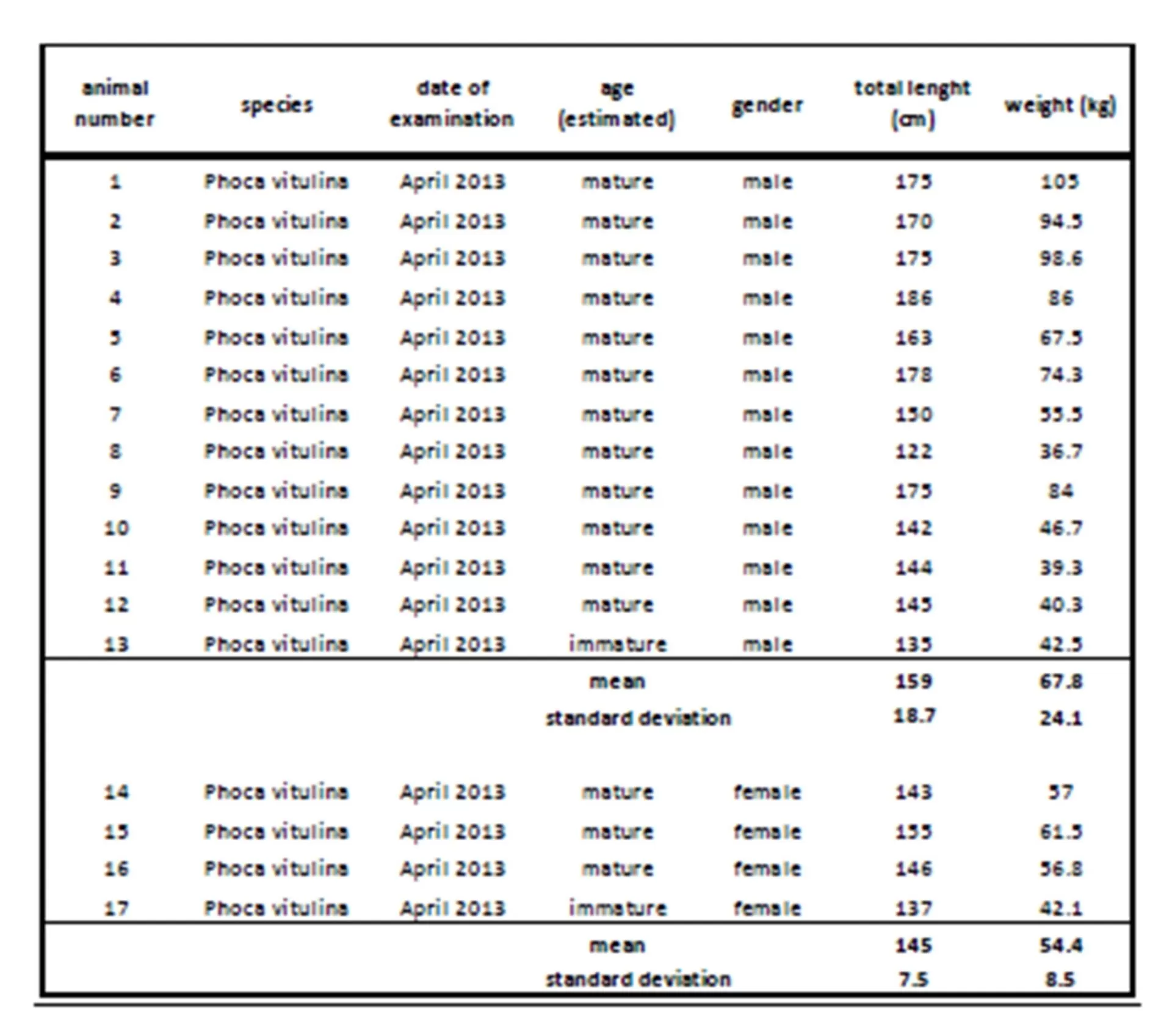
Correlation of cortisol concentration in serum, saliva and lachrymal fluid
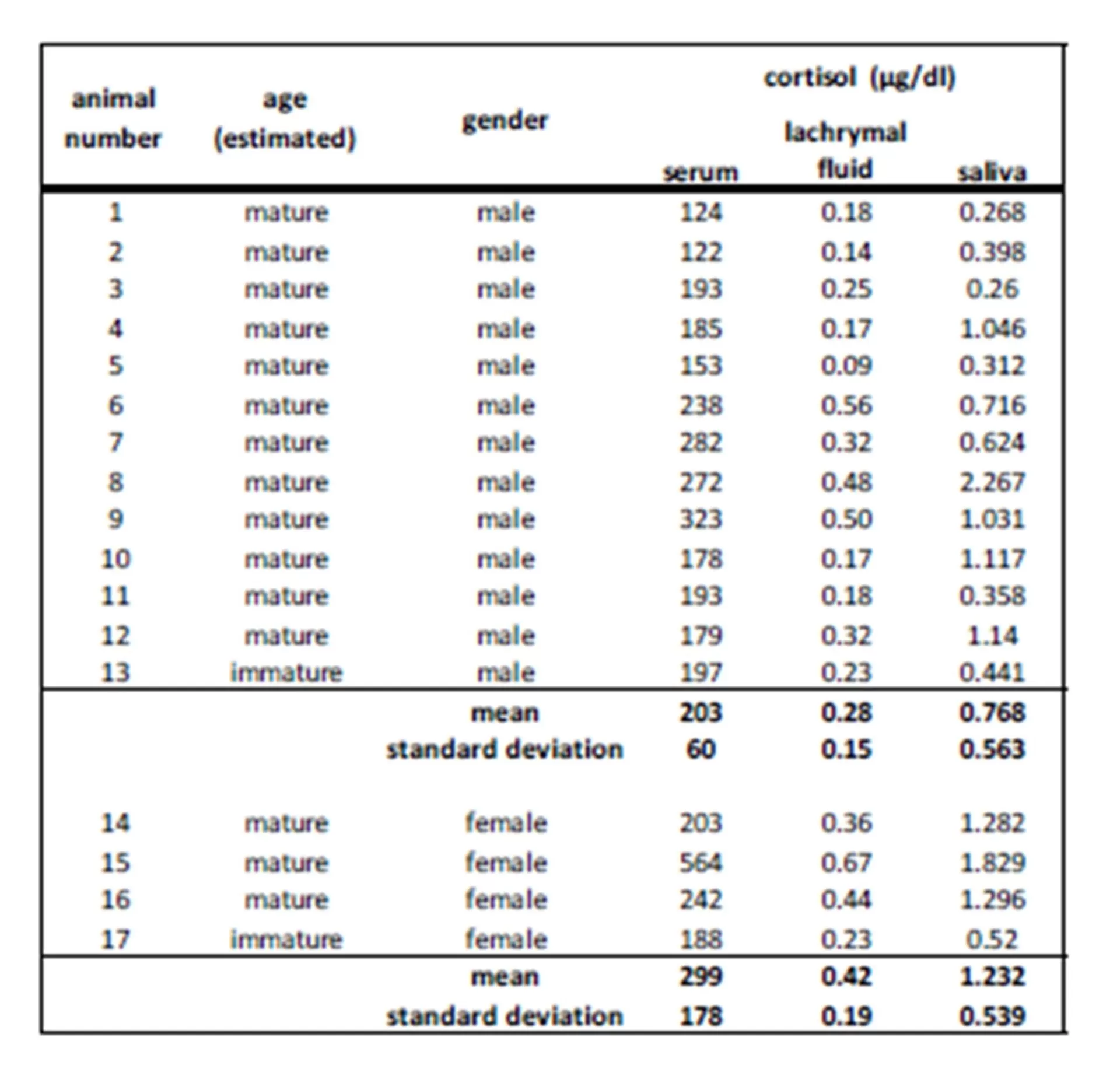
Figure 1-2 show the evaluation of regression analysis between serum cortisol and “free” cortisol concentrations in lachrymal fluid with R2 = 0.82 (p < 0.01) for linear correlation and R2 = 0.67 (p < 0.01) for exponential regression.
Comparing the concentrations of unbound cortisol in saliva and tears, the following correlation with R2 = 0.68 (p < 0.05) could be estimated. Regression analysis for saliva and serum cortisol concentration (R2 = 0.60, p < 0.05) is shown in figure 4.
Figure 1-2: Linear and exponential regression of lachrymal fluid and serum cortisol (µg/dl) in 17 harbor seals
Figure 3-4: Linear regression analysis of cortisol concentration (µg/dl) in lachrymal fluid, serum and saliva in 17 harbor seals
Discussion
Cortisol, released by the adrenal gland, is referred to as one of the main stress hormones to evaluate a stress response. Serious restraint and mild pain associated with venipuncture as the reference technique, however, is already sufficient for an increase of the stress hormone levels, especially for wild animals (Mormède et al., 2007, Cook et al., 2000). There have been several attempts to avoid or ease this influence by using indwelling catheters or habituation effects to serial sampling (Hopster et al., 1999) as well as training for voluntary husbandry behaviors, for example in harbor porpoises (Desportes et al., 2007). Nonetheless, a rapid sampling method of an appropriate material is needed for stress assessment in non-domestic animals.
Besides the mentioned difficulties of interpretation of blood cortisol concentrations, saliva, as an ultra-filtrate of blood, has the disadvantage that the matrix can be influenced by a possible contamination of food remains or blood. In addition, sampling from the oral cavity depends on the compliance of the animal in order to limit the risk for the examiner (Groeschl 2008, Sheriff et al., 2011). Lachrymal fluid was used in human research (Banbury 2009) and also evaluated as an alternative in a study of the effects of a mild stress challenge in dairy cattle (Khraim 2011). In this study, the author found evidence for painless sampling of tear fluid during lateral recumbency, bearing less risk of influencing cortisol concentration due to mental stress or stress related to pain (Khraim 2011). This discovery was also reflected in a significant correlation between serum and tear cortisol concentrations.
Certainly, the examination of wild animals, in comparison to farm animals, includes several difficulties. Aside from the lack of information of wild animals and its environment before and after temporal capture, missing experience of handling and trapping may exacerbate examination, sampling and the analysis (Sheriff et al., 2011). Hence, there is a need to confine possible pain- or stressful restraints and examination to a minimum. Consequently, we tried to find other methods and material to reduce the amount of invasive collected samples. So, in the course of the first inspection, lachrymal fluid was sampled from the conjunctival sack by soft- tissue swaps. Additionally, serum and saliva were sampled to compare the obtained data.
Cortisol was detected in all sampling materials (lachrymal fluid, saliva, blood) investigated in the present study.
The results of this study showed a clear increase in serum cortisol concentration in seven harbor seals (four males, three female). As earlier stated, trapping and fixation for investigative purpose may already cause an increase of cortisol concentration without receiving further information about possible stress condition before handling. This is also described in Eskesen’s (2009) stress research study investigating forty-five harbor porpoises trapped in pound nets.
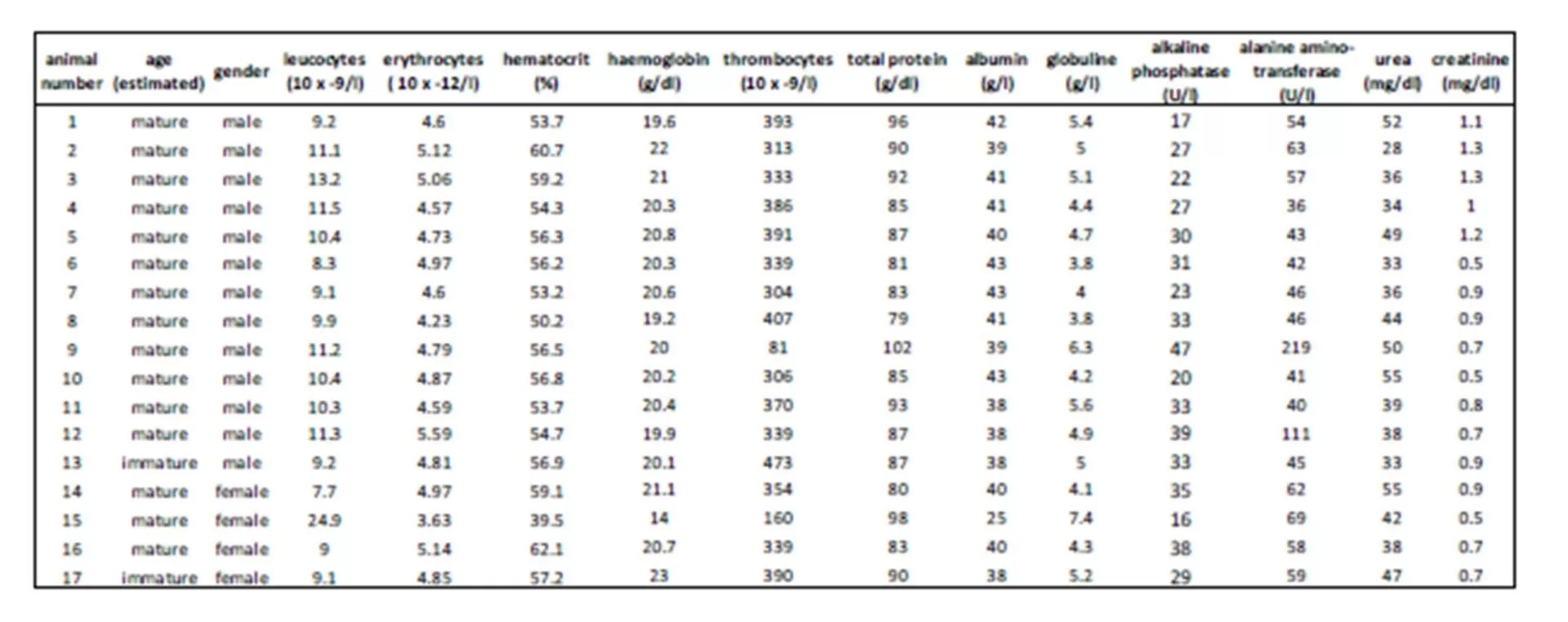
Still, possible interactions between glucocorticoids and the immune system have to be taken into account as the health status may also influence the level of cortisol (Fonfara et al., 2007, Desportes et al., 2007, Beineke et al., 2010). So an additional blood cell count and clinical chemistry were performed for further clarification. Here, the injured female seal showed the highest cortisol concentration combined with a slight rise in total protein (mean 88  6.5 g/l), indicating a possible infection. The decrease in hematocrit concerning this female as well as erythrocyte and thrombocyte numbers related to reference values may also be due to the skin wounds and a possible blood loss. This shows that illness and pain, combined with an activation of the immune and stress system, can cause an increase of cortisol via different pathways and interactions. Hence in this case, a clear distinction whether this gain in cortisol concentration results from the change in situation or the health status is not possible. Another likely explanation is that the cortisol concentration of this female seal already reached its maximum due to disease as a kind of ‘ceiling effect’ (Mellor et al., 2000) and so a further increase caused by trapping and handling could not be displayed.
Aside from serum cortisol, cortisol was also detected in saliva in the present survey, although regression analysis displayed only a moderate correlation between serum and saliva cortisol values. Determination of cortisol in saliva and also lachrymal fluid is limited to the free form of cortisol as saliva and tears are devoid of binding proteins. So, compared to plasma, saliva assays have to cope with a reduced sensitivity and specifity (Mormède et al., 2007) due to a lower cortisol concentration in saliva (Banbury 2009). Furthermore, a time lag between blood and subsequent changes in saliva values is also discussed in cortisol measurement in sheep and humans (Cook 2002). Perez et al., (2004) even described a possible variability between individuals within the same breed and age group in cattle.
Tear fluid reflected a good alternative to blood investigation in this study. In humans it could also be discovered that cortisol measurable in tear fluid had a proper correlation to blood cortisol (Banbury 2009). The regression analysis in our study displayed a reliable correlation between lachrymal fluid and serum cortisol results in harbor seals. Therefore, we concluded that changes of the hypothalamic-pituitary-adrenal (HPA) axis are also reflected by stress hormone changes in lachrymal fluid. Proof for this simultaneous increase of cortisol in different body fluid can also be found in the study of the effects of dexamethasone on the HPA — axis in cattle (Khraim 2011). Here, a suppression of HPA with this synthetic glucocorticoid led to a corresponding decrease of cortisol values in serum as well as in lachrymal fluid.
As the concentrations in lachrymal fluid of harbor seals were much less than in humans, the measuring range of the assay had to be adapted. Determination of low cortisol level implies certain difficulties which may result in an underestimated correlation between serum and lachrymal fluid results. This could also be seen in the study in dairy cattle of Khraim (2011). He deduced that taking samples from the conjunctival sack was less invasive as there was no increase in heart or respiration rate during this period. Furthermore, rapid performance of tear sampling reduces exogenous stimulation of HPA — axis, as it can be seen in catheterized animals (Cook et al., 2000), and so diminishes stress effects due to collection procedure. Consequently, lachrymal fluid sampling may function as a reliable and quick method for measuring cortisol concentration in wild harbour seals, too.
Conclusion
The examined group of free-ranging harbor seals consisted of animals in similar age and weight states, comparable to each other. Only one female showed obvious signs of illness. Cortisol could be detected in all investigated materials, with a significant correlation between cortisol in serum and tears. As far as the author’s know this is the first time such an approach was carried out in the investigation of stress reactions in wild harbor seals. Thus, lachrymal fluid may be a potential non- invasive alternative for stress the assessment of stress in harbor seals and this technique could be useful to reduce the fixation of animals for examination purposes. Nonetheless, a higher sampling number might be needed as well as a randomized study design in order to validate each method as less or even non-invasive.
Acknowledgements
The study was supported by the authorities for coastal protection, national parks and marine protection, Wadden Sea national park administration Toenning of Schleswig-Holstein, Germany. Thanks to the volunteers and staff members helping with the health monitoring program as well as the Endocrinology Laboratory.
References
1. Atkinson, S. (1997) “Reproductive biology of seals”. Reviews of Reproduction, 2(3), 175-194.
Publisher – Google Scholar
2. Banbury, L. K. (2009) “Stress biomarkers in the tear film.” PhD thesis, Southern Cross University, Lismore, NSW.
Google Scholar
3. Beineke, A., Siebert, U., Wohlsein, P. and Baumgaertner, W. (2010) “Immunology of whales and dolphins.” Veterinary Immunology and Immunopathology, 133(2), 81-94.
Publisher – Google Scholar
4. Cook, C. J., Mellor, D. J., Harris, P. J., Ingram, J. R. and Matthews, L. R. (2000) “Hands-on and hands-off measurement of stress.” The Biology of Animal Stress. CABI Publishing, 123-46.
5. Cook, C. J. (2002) “Rapid noninvasive measurement of hormones in transdermal exudate and saliva.” Physiology & Behavior, 75(1), 169-181.
Publisher – Google Scholar
6. Chrousos, G. P. (2009) “Stress and disorders of the stress system.” Nature Reviews Endocrinology, 5(7), 374-381.
Publisher – Google Scholar
7. Dierauf, L. A., and Gulland, F. M. (2010) CRC handbook of marine mammal medicine: health, disease, and rehabilitation. CRC press.
8. Desportes, G., Buholzer, L., Anderson-Hansen, K., Blanchet, M., Acquarone, M., Shephard, G., Brando, S. , Vossen, A. and Siebert, U. (2007) “Decrease stress; train your animals: The effect of handling methods on cortisol levels in harbour porpoises (Phocoena phocoena) under human care.” Aquatic Mammals, 33(3), 286.
9. Eskesen I.G., Geertsen B.M., Teilmann J., Desportes G., Riget F., Dietz R., Larsen F. and Siebert U. (2009) “Stress level in wild harbour porpoises (Phocoena phocoena) during satellite tagging measured by respiration, heart rate and cortisol.” Journal of Biological Society of the United Kingdom, 89(5), 885-892.
Publisher – Google Scholar
10. Evans, G. W. (1984) “Environmental stress.” CUP Archive, Cambridge, United Kingdom
Google Scholar
11. Fair, P. A., and Becker, P.R. (2000) “Review of stress in marine mammals.” Journal of Aquatic Ecosystem Stress and Recovery, 7.4, 335-354
Publisher – Google Scholar
12. Fonfara, S., Siebert, U., Prange, A., and Colijn, F. (2007) “The impact of stress on cytokine and haptoglobin mRNA expression in blood samples from harbour porpoises (Phocoena phocoena).” Journal of the Marine Biological Association of the United Kingdom, 87(1), 305-311.
Publisher – Google Scholar
13. Groeschl, M. (2008) ” Current status of salivary hormone analysis.” Clinical Chemistry, 54(11), 1759-1769.
Publisher – GoogleScholar
14. Haerkoenen, T., Dietz, R., Reijnders, P., Teilmann, J., Harding, K., Hall, A., Brasseur, S., Siebert, U., Goodman S.J., Dau Rasmussen, T. and Thompson, P. (2006) “The 1988 and 2002 phocine distemper virus epidemics in European harbour seals.” Diseases of aquatic organisms, 68(2), 115-130.
Publisher – Google Scholar
15. Hasselmeier, I., Fonfara, S., Driver, J., and Siebert, U. (2008) “Differential hematology profiles of free-ranging, rehabilitated, and captive harbor seals (Phoca vitulina) of the German North Sea”. Aquatic Mammals, 34(2), 149-156.
Publisher – Google Scholar
16. Hopster, H., Van Der Werf, J. T., Erkens, J. H. and Blokhuis, H. J. (1999) “Effects of repeated jugular puncture on plasma cortisol concentrations in loose-housed dairy cows.” Journal of Animal Science, 77(3), 708-714.
Google Scholar
17. Khraim, N. M. (2011) “Effects of Dexamethasone and Training on the Hypothalamic Pituitary Adrenal Response on Mild Stress Challenge in Dairy Cows.” PhD Thesis, University of Veterinary Medicine Hannover, Germany
18. Koolhaas, J. M., Bartolomucci, A., Buwalda, B., De Boer, S. F., Flügge, G., Korte, S. M., Meerlo, P., R. Murisong, R., Olivier, B., Palanzak, P., Richter-Levine, G., Sgoifok, A., Steimerj, T., Stiedl, O., van Dijkh, G., Wöhrd, M. and Fuchs, E. (2011) “Stress revisited: a critical evaluation of the stress concept.” Neuroscience & Biobehavioral Reviews, 35(5), 1291-1301.
19. Mellor, D. J., Cook, C. J., and Stafford, K. J. (2000) “Quantifying some responses to pain as a stressor.” The biology of animal stress: basic principles and implications for animal welfare, CABI Publishing, Wallingford, UK, 171-198.
20. Moestl, E., and Palme, R. (2002. “Hormones as indicators of stress.” Domestic Animal Endocrinology, 23(1), 67-74.
Publisher – Google Scholar
21. Mormède, P., Andanson, S., Aupérin, B., Beerda, B., Guémené, D., Malmkvist, J., Manteca, X., Manteuffel G., Prunet P., van Reenen, C.G., Richard S. and Veissier, I. (2007) “Exploration of the hypothalamic—pituitary—adrenal function as a tool to evaluate animal welfare.” Physiology & Behavior, 92(3), 317-339.
Publisher – Google Scholar
22. Mueller, S., Lehnert, K., Seibel, H., Driver, J., Ronnenberg, K., Teilmann, J., van Elk, C. Kristensen, J. Everaarts, E. and Siebert, U. (2013) “Evaluation of immune and stress status in harbour porpoises (Phocoena phocoena): can hormones and mRNA expression levels serve as indicators to assess stress?” BMC veterinary research, 9(1), 145.
Publisher – Google Scholar
23. Oki, C. and Atkinson, S. (2004) “Diurnal patterns of cortisol and thyroid hormones in the Harbor seal (Phoca vitulina) during summer and winter seasons.” General and Comparative Endocrinology, 136(2), 289-297.
Publisher – Google Scholar
24. Chacón Pérez, G., García-Belenguer Laita, S., Illera del Portal, J. C. and Palacio Liesa, J. (2004). “Validation of an EIA [enzyme immunoassay] technique for the determination of salivary cortisol in cattle.” Spanish Journal of Agricultural Research, 2(1), 45-51.
Publisher – Google Scholar
25. R Development Core Team 2011 “R: A Language and Environment for Statistical Computing” (Vienna: R Foundation for Statistical Computing) (www.R-project.org/)
26. Romero, M. L. and Butler, L. K. (2007) “Endocrinology of stress.” International Journal of Comparative Psychology, 20(2).
Google Scholar
27. Selye, H. (1950) “Stress, the Physiology and Pathology of Exposure to Stress.” Acta Medica
28. Selye, H. (1955) “Stress and disease.” The Laryngoscope, 65(7), 500-514.
Google Scholar
29. Sheriff, M. J., Dantzer, B., Delehanty, B., Palme, R. and Boonstra, R. (2011). “Measuring stress in wildlife: techniques for quantifying glucocorticoids.” Oecologia, 166(4), 869-887.
Google Scholar
30. Siebert U., Pozniak B., Anderson Hansen K., Nordstrom G., Teilmann J., van Elk N., Vossen A. and Dietz R. (2012) “Investigations of thyroid and stress hormones in free-ranging and captive harbor porpoises (Phocoena phocoena): a pilot study.” Aquatic mammals 37 (4), 443-453.
31. Swietlik, M. (2011) „Grundbegriffe der Umweltpsychologie: Stressoren (Klima, Hitze, Kälte, Gerüche, Schadstoffe).“ GRIN Verlag, Munich, Germany.
Publisher – GoogleScholar
32. Trites, A. W., and Pauly, D. (1998) “Estimating mean body masses of marine mammals from maximum body lengths.” Canadian Journal of Zoology, 76(5), 886-896.
Publisher – Google Scholar
33. Wright, A. J., Deak, T. and Parsons, E. C. M. (2009). “Concerns related to chronic stress in marine mammals.” Inter Whaling Commission, 1-7.
Google Scholar