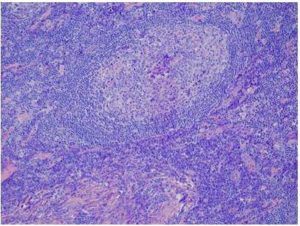
The Normal Results
RBC 5.00-5.40 10*12/L (4.30-5.70), leukocytes and their distribution, platelet count, reticulocytes, ferritin, interleukin- 1β (IL1-F2) 0.65 pg/ml (0.001-2.5), IgM, IgG2, IgG3, IgG4, IgA2, minerals, ceruloplasmin, urin analysis, complement C3 and C4, paraproteins, ASLO, uric acid, repeated glucose, fasting serum insulin level 42.38pmol/L (<56.00), vitamin B12, folic acid, lipoprotein (a) 0.14g/L (<0.20), thomocystein 10.3µmol/L (4.0-15.4). Renal, liver, pancreatic and gastroenterological (including malabsorption) sets, infection verification (causes), hematologic tests without anemia, autoimmune diseases and oncological set, no endocrinopathies. Imaging tests: the organs and cavities, neck by ultrasound, CT, MRI, PET/CT not detected another enlarged lymph nodes. In plasma: IL-1α, IL-2, IL-4, IL-10, IL-12/23, IL-17A, TNF alpha, IFN gamma, Th1/Th2 ratio. Mutational analysis: JAK2V617F, factor V Leiden (G1169A), factor II prothrombin (G20210A), MTHFR (C677T, A1298C)
Postoperative Results
The recheck in three weeks: ESR 10/34, RBC 5.47 x10*12/L, hemoglobin 127 g/L, hematocrit 0.388, MCV 71.00 fl, MCH 23.2 pg, MCHC 327.0 g/L, reticulocytes 0.72%, iron 10.2 µmol/L, transferrin 3.23 g/L, soluble transferrin receptor 6.65 mg/L, ferritin 141.59 µg/L, flow cytometry, fibrinogen 2.8 g/L, CRP 2.8 mg/L, interleukin-6 3.2pg/ml, procalcitonin 0.06 ng/ml, total cholesterol 5.00 mmol/L, HDL cholesterol 1.18 mmol/L. The recheck in twelve weeks: ESR 6/20, RBC 5.50×10*12/L, hemoglobin 13.6g/L, hematocrit 0,435, reticulocytes 0.93%, iron 16.2µmol/L, s. transferrin receptor 2.9 mg/L, fibrinogen 3.1 g/L, CRP 2.5mg/L, total cholesterol 3.21 mmol/L, HDL cholesterol 1.09 mmol/L, LDL cholesterol 1.57 mmol/L, triacylglycerols 0.55 mmol/L, interleukin-6 2.1 pg/ml, IgG 13.55g/L, IgG1 7.85g/L, IgA1 2120mg/L, IgD 90mg/L, IgE 115 IU/ml.
Discussion
The inflammation is a complex biological reaction to pathogenic and harmful stimuli. Autoinflammatory syndrome currently represents about nineteen different diseases. They are primarily and mainly mediated by interleukin- 1 family, especially IL-1β, Dinarello (2009). Clinical features, laboratory studies, genetics excluded the pediatric hereditary inflammatory syndromes, and other well-known immunological and inflammatory dysfunctions too. The exposure to an external stimuli (vaccine, adjuvant) prior to clinical manifestation, chronic fatigue and dry mouth were observed in patient according to the suggested major criteria for the diagnosis of ASIA. The new diseases including macrophagic myofasciitis, postvaccination phenomena and Gulf War Syndrome may be incorporated into autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld`s syndrome). A fever is not a striking feature of the autoinflammatory syndrome. Castleman disease must be taken into consideration by the differential diagnosis of well- defined enhancing mass in the abdomen, its unicentric type. Retroperitoneal localization is exceptional. The etiology is not known for sure. Diagnostic clue to this disease is an elevated concentration of IL-6 in plasma, chronic, iron-refractory mikrocytic anemia and positive PET/CT scan. The diagnosis should be confirmed by histological examination, and traditionally three histological variants are distinguished. Castleman disease is closely mimicked by IgG4-related sclerosing disease, in Jo et al (2011). The conclusive diagnosis in our patient was Castleman like disease that is presented as autoinflammatory syndrome. Vaccination can be other possible cause of lymph node hyperplasia, adjuvant related disease. It is very difficult exactly to determine the beginning of post-vaccination progressive process of the inflammation, because the clinical long-lasting latency period delays the indication of the laboratory studies and imaging tests. The process might start immediately post vaccination. Aluminum adjuvant stimulates Th2 type response and increases the synthesis of cytokines (IL-1, IL-4) as well as B/plasma cells production of IgG1, IgA1, IgD and IgE, in research study by Brewer et al. (1997). Aluminum alone has several pathologic effects such as impact on the erythropoiesis and is the agonist of the inflammasome- caspase cascade. The deposition of aluminum into tumor formation is one of the pathomechanisms, which occurs in this clinical observation. The determination of the non-physiological level of aluminum in patient`s lymphatic tumor is still unresolved and data about its amounts are difficult to interpret. The information retrieval may shed new light on the potential immunotoxicological role of aluminum- adjuvanted vaccines, Salemi et al (2010). Interleukin-1β along with interleukin-6 stimulate the induction of acute- phase response with subsequent production of the corresponded reactants by hepatocytes. The elevated levels of the interleukin-6 promote the large spectrum of clinical and mainly laboratory abnormal findings. Its increased concentrations cause an amplification of the inflammatory process and also accelerate the maturation of the plasma cells. Fibrinogen is a marker of inflammation rather than hemostasis. During the acute phase response serum levels of high-density lipoproteins drop via increased enzymatic hydrolysis of the lipoprotein. It is chiefly the effect of the endothelial lipase and secreted phospholipases A2. The inflammation causes a chronic anemia at different levels of erythropoiesis. The overproduction of hepcidin or its relatively high activity according to anemia impinges iron traffic leading to retention of the metal in the competent cells and thus to iron deficient erythropoiesis, Bergamaschi et al (2009). Interleukin-6 is the major inflammation- driver inducer of hepcidin synthesis and is also involved in differentiation of hematopoietic precursor cells, Nemeth et al (2004). The linkage between hepcidin and ferroportin blocks the transfer of iron from the duodenal mucosal cells into the circulation. This link increases the retention of iron within macrophages-inhibited recycling and deteriorates reutilization of iron by erythrophagocytosis. The pro-inflammatory cytokines have inhibitory effects on erythroid progenitor cells and reduce the expression of hematopoiesis growth factors, Weiss et al (2010). Alpha-1 antitrypsin inhibits the iron uptake into the erythroid progenitors.
Taken together, vaccines administration with aluminum adjuvant might be a potent stimulating factor on the immune/inflammation system that sometimes launches non-infectious systemic inflammation. Aluminum containing vaccines could initiate and promote the lymphoproliferative disease. This process enhances the synthesis and release of bioactive substances that provoked autoinflammatory syndrome. Blunting of the biological activity of erythropoietin along with the failure to properly utilize iron are the major causes of chronic anemia of inflammation. The pathogenesis of the post-vaccination phenomena is not completely understood, as well as its long-term health consequences. The vague symptoms and physical findings cause that in clinical practice the autoinflammatory syndrome is often not in time categorized into the accurately defined disease. The extirpation of the lymphoid tumor activating pro-inflammatory condition is the adequate kind of the treatment. In this patient follow-up exams are recommended for several years after the treatment was finished. More research and much greater knowledge are needed to clarify the relationship between aluminum adjuvant and the development of the autoinflammatory syndrome.
References
Agmon-Levin, N., Paz, Z., Israeli, E. & Shoenfeld, Y. (2009). “Vaccines and Autoimmunity,” Nature Reviews Rheumatology, 5 (11) 648-652.
Publisher – Google Scholar
Bergamaschi, G. & Villani, L. (2009). “Serum Hepcidin: A Novel Diagnostic Tool in Disorders of Iron Metabolism,”Hematologica, 94 (12) 1631-1633.
Publisher – Google Scholar
Brewer, J. M, & Alexander, J. (1997). “Cytokines and the Mechanisms of Action of Vaccine Adjuvants,” Cytokines, Cellular & Molecular Therapy, 3 233-46.
Publisher – Google Scholar
Dinarello, C. A. (2009) “Immunological and Inflammatory Functions of the Interleukin-1 Family,” Annual Review of Immunology, 27 519-50.
Publisher – Google Scholar
Jo, J.- H., Park, Y.- S., Jeon, Y. K., Nam, S. J. & Huh, J. (2011). ”Comparison of Plasma Cell Type of Castlemans Disease and IgG4-Related Sclerosing Disease: A Histopathological and Immunohistochemical Study,” Pathobiology 78(4) 227-32.
Publisher – Google Scholar
Molina, V. & Shoenfeld, Y. (2005). “Infection, Vaccines and Other Environmental Triggers of Autoimmunity,”Autoimmunity 38 (3) 235-245.
Publisher – Google Scholar
Nemeth, E., Tuttle, M. S., Powelson, J., Vaughn, M. B., Donovan, A., Ward, D. M., Ganz, T. & Kaplan, J. (2004). “Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing its Internalization,” Science, 306 (5704) 2090-2093.
Publisher – Google Scholar
Salemi, S. & D’Amelio, R. (2010). “Vaccination and Autoimmune Diseases,” International Reviews of Immunology, 29 (3) 239-246.
Publisher – Google Scholar
Shoenfeld, Y. & Agmon- Levin, N. (2011). “‘ASIA’- Autoimmune/Inflammatory Syndrome Induced by Adjuvants,” Journal of Autoimmunity, 36 (1) 4-8.
Publisher – Google Scholar
Siegrist, C.- A. (2007). “Autoimmune Diseases after Adolescent or Adult Immunization: What Should Expect?,” Canadian Medical Association Journal, 177 (11) 1352-1354.
Publisher – Google Scholar
Weiss, G. & Gasche, C. (2010). “Pathogenesis and Treatment of Anemia in Inflammatory Bowel Disease,” Hematologica, 95 (2) 175-178.
Publisher – Google Scholar
Wraith, D. C., Goldman, M. & Lambert, P.- H. (2003). “Vaccination and Autoimmune Disease: What is the Evidence?,”Lancet, 362 (9396) 1659-1666.
Publisher – Google Scholar