Introduction
The issues of the efficiency improvement are among the most relevant and key in the economy. Among the main approaches to the definition of the concept of “efficiency” one can distinguish such approaches as resource-costly, efficient, resource-reserve, complex, etc. According to the author, the integrated approach allows us to consider the concept of “efficiency” most widely. Therefore, this approach was used to develop the CDMO efficiency improvement mechanism.
R&D activities (for new drug development) are characterized by such features as high uncertainty, long-term projects (up to several years), a high risk of negative results, the participation of specialists of various fields of knowledge, the complexity of completing a project on time, the need for many reports and documentation (DiMasi et al., 2016, Scannel et al., 2012, Schultz, 2013).
In the modern world, a business directly related to R&D faces many challenges. The main of which are protection of intellectual property, high competition, choice of effective management approaches, changing socio-economic environment, search and retention of highly qualified personnel. Therefore, the concept of this study was to create an easy-to-implement and easy-to-use efficiency improvement mechanism for all CDMOs; such mechanism will contribute to their organic growth and long-term development.
Materials and Methods
The methodological basis of the research: the author used such scientific methods as abstract-logical in the study of the economic essence and nature of CDMO activities; scientific observation and comparative analysis to study the current state and development of CDMOs. Such approaches were applied as systematic (to set goals and to define research objectives, to develop the CDMO efficiency improvement mechanism) and integrated (to develop the classification of CDMO services, the efficiency evaluation method and the CDMO risk assessment method). The author used a graphical method to present the results of the study.
The information and statistical base of the research: official statistical and analytical materials of the FDA, the PhRMA, the EMA, the EFPIA, the international consulting companies as McKinsey, PricewaterhouseCoopers, Booz Allen Hamilton; various materials of scientists on the problems of the research; statistical and analytical materials of such companies as Merck, Lonza, Catalent, Siegfried Group, Patheon, Institute of Pharmaceutical Technologies, ChemRar and etc.
Results
The author conducted the research on the activities of CDMOs in different countries. In the course of this study, the general classification of services offered by CDMOs was proposed. The author takes the life cycle of the drug as its basis. The simplified drug life cycle (Figure 1) includes the stages of its development, research, registration, production and sale.

Fig 1. Simplified drug life circle
Source: developed and compiled by the author
Figure 2 shows the classification of CDMO services. The full range of services consists of the basic, related and additional services.
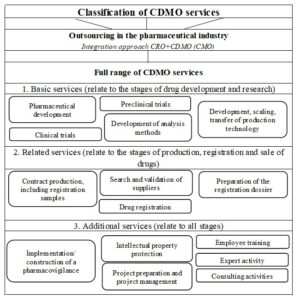
Fig 2. Classification of CDMO services
Source: developed and compiled by the author.
The basic services (related to the stages of drug development and research) include pharmaceutical development services, services for the preclinical and clinical trials, services for the development of analysis methods, services for the development, scaling, transfer of production technology.
Among the related services (related to the stages of production, registration and sale of drugs), the author identifies services for contract production, including registration samples, services for searching and validating suppliers, services for preparing a registration dossier for drugs, services for state registration of drugs.
The additional services somehow relate to the entire life cycle of the drug. This group of services includes services for the implementation/construction of a pharmacovigilance system, intellectual property protection services, project preparation and management services, consulting and expert services, as well as personnel training services.
The author defines a pharmaceutical development service as a type of entrepreneurial activity, during which, on the instructions of the customer (sponsor), a complex of studies is carried out to search for new pharmacologically active substances, study their medicinal properties, develop technologies for the production of pharmaceutical substances, develop formulations and technologies for the production of drugs.
Further, the general pharmaceutical development algorithm is proposed (Figure 3). This algorithm is based on the project approach and includes the stages of initiation, planning, execution, control and completion.
Then the CDMO efficiency improvement mechanism is proposed (Figure 4). This mechanism includes the following components: the subject of regulation, the object of regulation, the purpose of regulation, methods and tools of regulation.
The subject of regulation is a CDMO. The object of regulation is its activity. The CDMO management sets a goal – to increase the efficiency of CDMO services. Then one formulates the qualitative and quantitative characteristics of the goal (Cee, Coe, CE). The author suggests using the following methods and tools to achieve the goal.
The project approach is used as the main tool to provide the CDMO services. The process approach is proposed to be used as an operational basis for the management of such departments as financial and economic, legal, marketing, logistics, IT, HR, procurement and primary customer service.
Under force majeure circumstances (for example, in conditions of increasing instability of the socio-economic and/or political situation, etc.), it is proposed to use the situational approach, which reflects the principle of a systematic approach that any company is an open system that constantly interacts with the external environment.
The main task of the manager is a deep understanding of the company’s activities and the timely application of one or another approach, and not the opposition and separation of these approaches to management.
The competent implementation of the kaizen approach, originally developed at the Japanese Toyota enterprise, has shown its effectiveness in many companies around the world (Imai, 2012, Maurer, 2014, Rother, 2018). It is important to draw attention to the fact that this is, first of all, the philosophy of the employee’s attitude to his work, to the company, to his life in general. The author suggests using the following elements of the kaizen approach for CDMOs. It is proposed to use the CDMO Kaizen Declaration (Table 1) for each employee, starting from the top management, ending with ordinary employees.
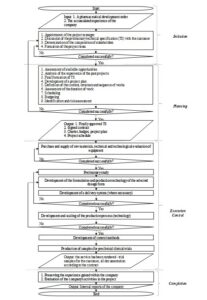
Fig 3. General pharmaceutical development algorithm
Source: developed and compiled by the author.
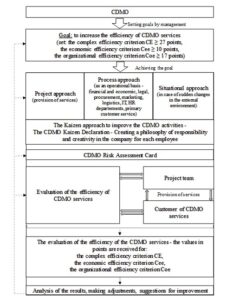
Fig 4. CDMO efficiency improvement mechanism
Source: developed and compiled by the author.
Table 1: the CDMO Kaizen Declaration
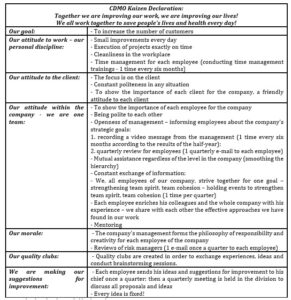 Source: developed and compiled by the author.
Source: developed and compiled by the author.
The sense of the kaizen approach is to create and develop a philosophy of responsibility and creativity in the CDMO for each employee.
The CDMO Kaizen Declaration includes such items as a common goal, attitude to the work of an employee, attitude to the client, attitude between employees within the company, morale, organization of quality clubs and making suggestions for improvement. And the implementation of this declaration, of course, should begin with the company’s management.
The pharmaceutical market at the present stage is highly competitive. Therefore, the company aims not to beat or destroy competitors at any cost but to increase the number of customers with its friendly and polite attitude to them, fulfilling orders efficiently and on time. The focus of attention is not directed to the fight against competitors, but to customer satisfaction.
The essence of the kaizen philosophy is to make small improvements, but every day. It is proposed to conduct time management trainings 1 time per half year in order to increase the professional and personal effectiveness of each employee. Because the time resource in the field of new drug development is very important.
CDMO management is increasingly faced with the issue of recruiting and retaining highly qualified employees. For scientists, it is important not only fair payment for the intellectual work but also self-realization. Therefore, the management needs to create a friendly atmosphere in the company and provide opportunities for self-realization.
It is proposed to emphasize the importance of each employee for the company to strengthen the team spirit and to pay attention to how useful it is to be polite to each other in work. Very often large and medium-sized companies are characterized by closeness, alienation of management from the rest of the company’s employees. And this negatively affects the company and its further development. Therefore, it is proposed to smooth out the hierarchy by increasing the openness of management and focusing on the principle of mutual assistance between employees at all levels. If employees know which direction to move in and know that their efforts will be rewarded financially and morally, they will work more efficiently.
Management can inform employees about strategic goals using video messages (once every six months, based on the results of the half-year), personal e-mailing to each employee based on the results of the quarter. It is necessary to provide a constant exchange of information between employees. Each employee can enrich other colleagues with his experience. As a result, both employees and the whole company win. It is proposed to hold events once a quarter to strengthen team spirit, team cohesion. It is also proposed to introduce a mentoring institute. Every company has people who have huge professional and personal experience and are ready to share this experience with young colleagues.
The morale of employees will be strengthened by specially prepared reviews of professional risk managers of the company. Such reviews can highlight the current situation from different sides avoiding negative moods. One can send such review by e-mail to each employee 1 time per quarter.
Quality clubs are created in each area of the company’s activity in order to exchange experience, ideas and to conduct brainstorming sessions. Every employee should be involved in the quality clubs. The activity of such clubs is carried out on an ongoing basis which contributes to the improvement of the psychological atmosphere in the team. Thanks to such quality clubs, it becomes clearer where to look for innovative solutions, with whom to cooperate outside the company, so that it is easier to find innovative solutions. It is suggested making proposals to improve the work of the company by each employee to his chief 1 time per quarter. The manager processes the received information and then holds a quarterly meeting to discuss all proposals and ideas. The most successful of them are sent to the higher management. It is necessary to pay special attention that every idea should be fixed. No matter how strange it may seem at first.
The application of the kaizen approach (cultivating the philosophy of responsibility and creativity in the company) will subsequently help the management to use project, process and situational approaches to management more effectively.
CDMO’s business is highly risky. So, it is proposed to assess the risks and develop a list of actions necessary to reduce them. The author suggests the following method to assess the risks. One fills out a CDMO risk assessment card. The author presents 41 risks faced by CDMOs in the internal and external environment: industrial, financial, political, natural, social, entrepreneurial, etc. in this card. It is proposed to assess the probability of occurrence and the degree of danger of each of these risks (Table 2). The degree of danger is expressed by the share of profit that will be jeopardized in the event of an unfavorable development of events. It is necessary to determine the loss of the profit share in the event of a risk.
Table 2: assessment of the probability of occurrence and the degree of danger of the risk
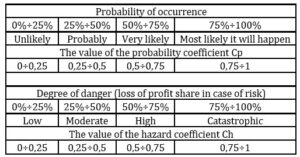
Source: developed and compiled by the author.
Each risk receives an assessment in the form of a decimal coefficient calculated according to the formula (1).
Cp – the probability coefficient, Ch – the hazard coefficient.
If the value of the risk assessment is from 0 to 0,2 – the risk is considered as insignificant (I); from 0,2 to 0,5 – as moderate (М); from 0,5 to 1 – as critical (С). Then one includes in the card those actions that will be carried out to reduce risks. First, an action plan is drawn up to reduce critical risks, then for moderate ones and at the end for minor ones. The CDMO risk assessment card was developed for one of the Swiss CDMOs (Table 3).
Table 3: CDMO risk assessment card
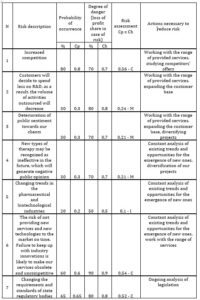
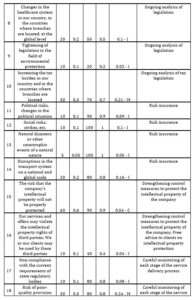
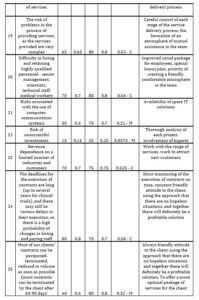
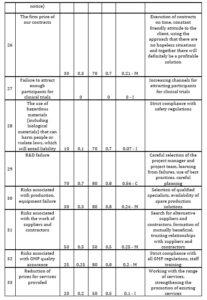
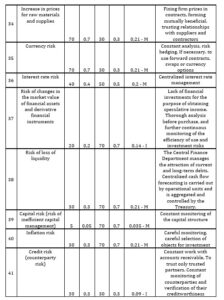
Source: developed and compiled by the author.
The author suggests the following evaluating CDMO efficiency method for the mechanism. The economic efficiency criterion and the organizational efficiency criterion are proposed for evaluation of CDMO efficiency (Figure 5) because organizational efficiency and economic efficiency are closely interrelated.
Economic efficiency refers to the ratio of the obtained economic result (effect) and the costs that were aimed at obtaining it (Cokins, 2017). At its core, it shows how successfully (effectively) the activities of a household, company, state, are carried out, whether this activity is profitable.
The profitability indicator is used to assess economic efficiency. Profitability indicators are relative indicators and comprehensively show the efficiency of using the resources at the disposal of the company (financial, material, human resources, etc.) from different sides.
The author suggests using the coefficients shown in Figure 5 as indicators of the economic efficiency. Formulas for their calculation are presented in Table 4. The higher the profitability indicators, the more efficiently the company uses its resources.
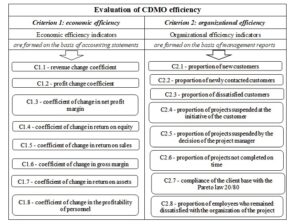
Fig 5. Evaluation of CDMO efficiency
Source: developed and compiled by the author.
Table 4: indicators for assessing the CDMO economic efficiency
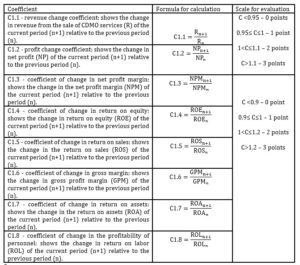
Source: developed and compiled by the author.
The more efficiently the company is organized, the more it can attract new customers and retain existing ones. The more stable the company and its growth, the more satisfied customers and employees it will have. This is especially true in the field of drug development, where each order received by a CDMO is a unique project. The author suggests using the coefficients shown in Figure 5 as indicators of the organizational efficiency. Formulas for their calculation are presented in Table 5.
It is proposed to use the questionnaire method to identify dissatisfied customers. When the order has already been completed, the client is invited to fill out a small questionnaire and share his opinion, vision, answering a few questions. The project manager will be able to assess the strengths and weaknesses of his work and the work of the project team and draw conclusions for the future about what needs improvement, optimization, etc. It is proposed to use the questionnaire method to determine the degree of satisfaction of employees involved in the implementation of the project. At the end of the project, employees are invited to fill out a short questionnaire, to answer a few questions, to share their opinions, suggestions, visions, ideas. The questions for this questionnaire are prepared by the project manager.
The project manager will be able to assess how well the team was formed (professional, personal qualities, psychological atmosphere in the team), as well as evaluate his work and the work of the team as a whole, get feedback, new ideas, draw conclusions for the future. The author suggests using a point system to evaluate each coefficient. It is the most visual and convenient.
The economic efficiency criterion Cee is calculated according to the formula 2 in points.
The organizational efficiency criterion Coe is calculated according to the formula 3 in points.
The complex efficiency criterion CE is calculated according to the formula 4 in points.
Table 5: indicators for assessing the CDMO organizational efficiency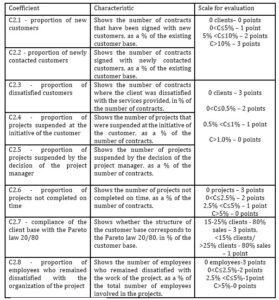
Source: developed and compiled by the author.
The economic efficiency criterion and the organizational efficiency criterion have the same weight in the formation of the complex efficiency criterion CE. CDMO efficiency is considered low if the value of the CE is from 0 to 14 points; it is considered satisfactory if the value of the CE is in the range from 15 to 26 points; it is considered high if the value of the CE is 27 or more points (Table 6)
Table 6: scale for evaluating CDMO efficiency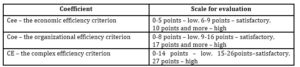
Source: developed and compiled by the author.
The economic and organizational CDMO efficiency can be analyzed separately. This is the advantage of this method. The efficiency of the same Swiss CDMO was analyzed using this method. The calculation of the economic efficiency criterion Cee, the organizational efficiency criterion Coe and the complex efficiency criterion CE for this company is presented in Figure 6.
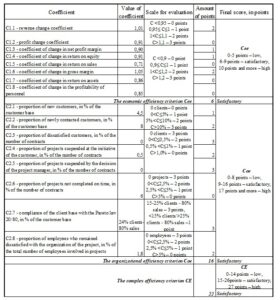
Fig 6. Evaluation of the efficiency of the Swiss CDMO
Source: developed and compiled by the author.
The calculation shows that CDMO efficiency of this company is estimated as satisfactory in 2021. Due to the high organizational efficiency, in 2022 there are all chances to have a high rating for the CDMO efficiency.
According to the evaluating CDMO efficiency method proposed by the author, the qualitative characteristic for the set goal of the CDMO efficiency improvement mechanism will be the complex efficiency criterion CE calculated according to formula 4. Table 6 shows the possible quantitative values of these criteria – the complex efficiency criterion CE should be equal to 27 points and higher; the economic efficiency criterion Cee should be 10 points and higher; the organizational efficiency criterion Coe should be 17 points and higher. The evaluation of the CDMO efficiency is done after an order is completed. Further the results are analyzed, the necessary adjustments and suggestions for improvement are made. The efficiency of this Swiss CDMO was evaluated in the case of using the proposed mechanism. The data obtained are presented in Figure 7.
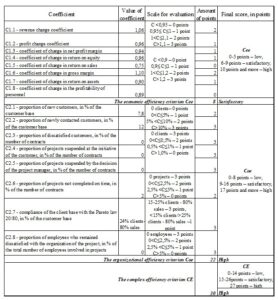
Fig 7. Evaluation of the efficiency of the Swiss CDMO after using the mechanism proposed by the author
Source: developed and compiled by the author.
The implementation of the proposed mechanism is able to ensure stable increase in the CDMO efficiency. Stable annual growth of 5% of economic efficiency coefficients C1.1÷C1.8, stable growth of new and newly applied customers by about 5%, stable decrease in the number of projects not completed on time by about 5%, high loyalty of customers and employees of the company are predicted.
One can see in figure 7 that using this mechanism the economic efficiency criterion Cee will increase and will be equal to 8 points; the organizational efficiency criterion Coe will also increase and will be equal to 22 points; the complex efficiency criterion will be equal to 30 points, which corresponds to the rating “high”. One can see that the CDMO efficiency increases by using the mechanism proposed by the author.
Conclusion
The CDMO efficiency improvement mechanism is a set of methodological, conceptual and organizational solutions that ensure the improvement of CDMO efficiency and contribute to the long-term development of such companies.
The proposed mechanism is cyclical and includes feedback. The opinions of both its employees and customers are taken into account in the work of the company.
This mechanism is easy to implement and is available to use for any CDMO. The above mechanism for increasing efficiency will contribute to the stable organic growth of CDMO and its long-term development.
The CDMO efficiency improvement mechanism is intended for CDMO management, which aims to increase the efficiency of the company and attract more customers.
References
- Cokins, G. (2009), Performance Management: Integrating Strategy Execution, Methodologies, Risk, and Analytics, Wiley, Hoboken, New Jersey.
- Cokins, G. (2017), Strategic Business Management: From Planning to Performance, Wiley, Hoboken, New Jersey.
- Contract Pharma (2018). ‘Integrating CDMO and CRO Services’. [Online], [Retrieved April 10, 2022], https://www.contractpharma.com/contents/view_online-exclusives/2018-09-04/integrating-cdmo-and-cro-services/
- DiMasi, J., Grabowski, H. and Hansen, R. (2016), ‘Innovation in the pharmaceutical industry: New estimates of R&D costs’, Journal of Health Economics, 47, 20-33.
- Imai, M. (2012), Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy 2/E, McGraw-Hill Professional, New York, NY.
- Grabowski, H., Long, G., Mortimer, R. and Boyo, A. (2016), ‘Updated trends in US brand-name and generic drug competition’, Journal of Medical Economics, 20, 1-9.
- Maurer, R. (2014), One Small Step Can Change Your Life: The Kaizen Way, Algonquin Books (division of Workman), New York, NY.
- Maurer, R. (2012), The Spirit of Kaizen: Creating Lasting Excellence One Small Step at a Time, McGraw-Hill Professional, New York, NY.
- Moorcroft, M. (2017) ‘History Guides Future CMO Strategies’, Pharmaceutical Technology, 41(7), 80-81.
- Rother, M. (2018), The Toyota Kata Practice Guide: Practicing Scientific Thinking Skills for Superior Results in 20 Minutes a Day, McGraw-Hill Education, New York, NY.
- Scannell, J.W., Blanckley, A., Boldon, H. and Warrington B. (2012) ‘Diagnosing the decline in pharmaceutical R&D efficiency’, Nature reviews Drug discovery, 11(3), 191-200.
- Schultz, T. (2013) ‘Turning healthcare challenges into big data opportunities: a use-case review across the pharmaceutical development lifecycle’, Bulletin of the American Society for Information Science and Technology, 39(5), 34-40.



















