Introduction
Venous thromboembolism is one of the major causes of mortality after surgery and one of the strongest patient-specific risk factors is obesity, a factor rapidly becoming more prevalent. Obese individuals have an increased percentage of fat per kilogram bodyweight and blood flow in adipose tissue is lower compared to lean body mass (Cheymol, 2000). Thus, proper dosing regimens need to be established.
Pharmacological thromboprophylaxis is widely used, and recommendations for peak and through levels of the substitute end-point “anti-factor Xa-activity” (anti-Xa) have been given (Levine et al., 1989). Renal function and thus elimination has been taken into account in several studies, but rarely body weight/obesity. The recommendations of the Medical Products Agency in Sweden, give risk classification of the patient, renal function and patient age as the variables that indicate dose adjustments (FASS, 2013). Only recently body weight regarding standard thromboprophylaxis for the morbidly obese has been added in Swedish and American PDR (FASS, 2013, PDR, 2013).
In a previous study, we measured anti-Xa after fixed doses in patients with varying body weights, and found a negative linear correlation with body weight. Neither the peak activity nor the area-under-curve observed in the normal-weight subjects were reached in the obese, and we concluded that the recommended doses were inadequate in heavy patients (Frederiksen et al., 2003).
The objective of the present study was to test the hypothesis that, by using a weight-dependent dose it would be possible to get the same anti-Xa activity in obese patients as in normal-weight subjects.
Patients and Methods
Ten patients scheduled for gastric bypass surgery were recruited after informed consent. Six were women, four were men. All invited participants concluded the study. Body weights ranged between 108 and 147 kg (median 127 kg); Body mass indices ranged between 35 and 58 kg/m2. No patient was planned for surgery within the next two-week period.
Each of our subjects was tested twice, the minimum interval between injections was set at five days and the order of injections was randomised. At each test a subcutaneous dose of enoxaparin (Klexane®, Roerig, Sweden) was given. As estimated from data from our former study, (Frederiksen et al., 2003) the tested lower dose (0.3 mg/kg) corresponds to the standard dose recommendation for low-risk patients of 20 mg, while the higher dose (0.6 mg/kg) corresponds to the 40 mg recommended standard dose for high risk patients.
Blood samples for anti-Xa were drawn before enoxaparin-injection and at one-hour intervals up to six hours post-injection, then at eight and ten hours. Samples were collected in tubes with 0.5 ml 0.129 mmol/l sodium citrate solution. Tubes were immediately centrifuged at 2000g for 20 minutes; plasma was separated and frozen at – 80° C. After all subjects had been tested twice, samples were analysed in a batch using the Behring Coagulation System (Behring Diagnostica AB, Stockholm Sweden) and anti-Xa was measured using Coamatic® Heparin (Chromogenix Instrumentation Laboratory S.p.A., Milano, Italy).
Some individuals had a (low) spontaneous anti-Xa at baseline, as previously described (Bendz et al., 1999). We subtracted this activity from all further samples from these individuals; incremental values were used in the further analyses.
Data were stored in Microsoft Excel® and analysed using Graph Pad Prism 5. The study was approved by the local Ethics Committee in Lund.
Results
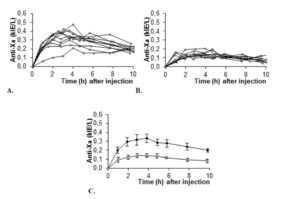
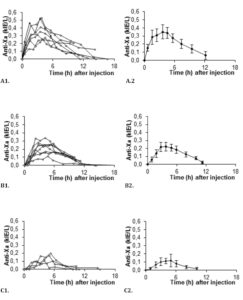
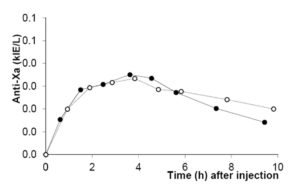
All ten subjects completed the study. Anti-Xa was plotted for the two different doses as a function of time for individual patients as well as for the group (summarized in figure 1). Anti-Xa peaked after approximately four hours. Peak values for 0.6 mg/kg were twice those for 0.3 mg/kg and the difference was statistically significant at all time-points.
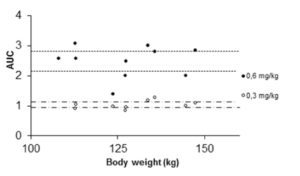
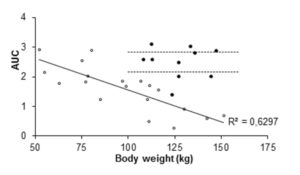
Figure 5: Area under curve (AUC) values for individual patients given 40 mg enoxaparin (open circles and trend line). Closed circles represent AUC values for individual patients given 0.6 mg/kg enoxaparin. Dashed lines mark 95% CI for AUC-means in the 0.6 mg/kg group. The 40 mg data are from our previous study (Frederiksen et al. 2003, reprinted with permission).
Discussion
With increasing prevalence of obesity in the population, all surgeons need proper regimens to handle the weight-specific problems arising. Thromboembolic disease is a feared complication to surgery. Bariatric surgical procedures are associated with a 30-day overall mortality rates of 0.1—2% (Buchwald et al., 2004, Flum et al., 2005). The large Scandinavian study SOReg reports an even lower 90 day figure, 0.04% (11/25038) (Stenberg et al, Annals of Surgery, In press) The incidence of postoperative deep venous thrombosis or symptomatic venous thromboembolism has been stated to be 0.8—2.4% (Westling et al., 2002, Prystowsky et al., 2005, Podnos et al., 2003, Eriksson et al., 1997). Retrospective analyses have reported an incidence of fatal pulmonary embolism after obesity surgery of 0.2—0.3% (Eriksson et al., 1997, Sapala et al., 2003). The American Society of Bariatric Surgery Registry has given pulmonary embolism as the most common cause of 30-day postoperative death and states that prophylaxis is recommended for all patients and should be continued until patients are ambulatory. The following guidelines have been given by the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery: Sequential compression devices and subcutaneous unfractionated heparin 5000 IU or LMWH should be initiated shortly (within 30—120 min) before bariatric surgery and repeated every 8—12 h postoperatively until patient is fully mobile. IVC filter is suggested to be reserved for higher risk obese patients (e.g., pulmonary hypertension or hypercoagulable state) (Mechanick et al., 2008). Even with thromboprophylaxis obese patients are at higher risk of thrombosis (Rocha et al., 2006).
Although laparoscopy causes less surgical trauma compared to open surgery (Schauer and Sirinek 1995), laparoscopy has been associated with reduced venous return and thereby possibly increased risk of thrombosis due to pneumoperitoneum and reversed Trendelenburg position (Nguyen et al., 2003).
LMWHs are normally given in either of two standard doses, the choice of which is determined by assessment of patients being at normal or at high risk of thromboembolic disease. Dosing intervals have been discussed in ICU patients, but standard practice in elective abdominal surgery, is once every 24h.
The recommended doses of LMWH stem from large, well-designed studies not commenting the effect on morbidly obese patients being performed in a bariatric surgery setting (Kakkar et al., 1993, Group 1997). The proper dosage in heavy-weight patients is still not scientifically determined, although different fixed doses have been compared in the obese setting (Simone et al., 2008, Rowan et al., 2008).
Given the relative rarity of pulmonary embolism, a study comparing fixed to weight-related dosing of LMWH, needs a substitute endpoint. Anti-Xa has been accepted as that endpoint, although questioned on correlation with thromboembolic complications in a paper on ICU-patients (Rutherford et al., 2005). Steady-state enoxaparin activity levels are well predicted by single-dose pharmacokinetics and peak and through values for desired activity are known. We have established plasma anti-Xa values for our laboratory in normal weight patients given recommended doses (Frederiksen et al., 2003). The values so obtained are taken to be the desired range. In the present set of experiments we compared the effect on plasma anti-Xa of two weight-adjusted dosing regimens.
In order to strengthen statistics, the study was designed to enable each patient to serve as its own control for the weight-adjusted two doses. Laboratory accuracy was enhanced by performing a batch analysis of deep frozen samples. Although the number of patients is small, differences in anti-Xa between doses are large. For all patients the 0.6 mg/kg AUC-values fell within the reference range established for our laboratory when giving 40 mg enoxaparin once daily to normal-weight patients (fig 5). The dose of 0.3 mg/kg body-weight for obese patients did not reach the reference values, neither for peak activity (fig 1 and 3), nor for AUC values (fig 2 and 5).
Since regimens with fixed doses every 24h are standard practice in abdominal surgery, our study could suggest that new recommendations for dosing LMWH may be indicated in obese patients. Our findings are clearly supported by a recent study on morbidly obese, medically ill patients (Rondina et al., 2010). The present data were recruited in obese patients, who were heavy for body height but also heavy in absolute terms. Further studies are indicated to see whether the implications of this study can be transferred to heavy, non-obese patients.
In summary obese patients do not reach the same level of thromboprophylaxis as normal weight subjects, if standard fixed doses of LMWHs are given, at least not as measured using the surrogate variable anti-factor Xa. For enoxaparin, a weight-adjusted regimen of 0.6 mg/kg body-weight is suggested but clinical studies in the perioperative setting are needed to assess the risk of post-operative bleeding.
Acknowledgements
Financial support was received from The Obesity Research Foundation, Department of Surgery at Skåne University Hospital Lund, the Sparre foundation, the Crafoord foundation and from Roerig Sweden. We gratefully acknowledge the expert assistance from the nursing staff of the Obesity clinic in Landskrona.
References
1.Bendz, B., Hansen, J.B., Andersen, T.O., Ostergaard, P. & Sandset, P.M. (1999) Partial depletion of tissue factor pathway inhibitor during subcutaneous administration of unfractionated heparin, but not with two low molecular weight heparins. Br J Haematol, 107(4), 756-62.
Publisher – Google Scholar
2.Buchwald, H., Avidor, Y., Braunwald, E., Jensen, M.D., Pories, W., Fahrbach, K. & Schoelles, K. (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA, 292(14), 1724-37.
Published – Google Scholar
3.Cheymol, G. (2000) Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet, 39(3), 215-31.
Publisher
4.Eriksson, S., Backman, L. & Ljungstrom, K.G. (1997) The incidence of clinical postoperative thrombosis after gastric surgery for obesity during 16 years. Obes Surg, 7(4), 332-5; discussion 336.FASS 2013:http://www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=19891208000057&DocTypeID=3&UserTypeID=0. Vol. 2013. Last accessed Nov 18, 2013.
5.Flum, D.R., Salem, L., Elrod, J.A., Dellinger, E.P., Cheadle, A. & Chan, L. (2005) Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA, 294(15), 1903-8.
Publisher – Google Scholar
6.Frederiksen, S.G., Hedenbro, J.L. & Norgren, L. (2003) Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg, 90(5), 547-8.
Publisher
7.Group, E.S. (1997) Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. . Br J Surg, 84(8), 1099-103.
Publisher
8.Kakkar, V.V., Cohen, A.T., Edmonson, R.A., Phillips, M.J., Cooper, D.J., Das, S.K., Maher, K.T., Sanderson, R.M., Ward, V.P. & Kakkar, S. (1993) Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. The Thromboprophylaxis Collaborative Group. Lancet, 341(8840), 259-65.
Publisher
9.Levine, M.N., Planes, A., Hirsh, J., Goodyear, M., Vochelle, N. & Gent, M. (1989) The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparine low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb Haemost, 62(3), 940-4.
10.Mechanick, J.I., Kushner, R.F., Sugerman, H.J., Gonzalez-Campoy, J.M., Collazo-Clavell, M.L., Guven, S., Spitz, A.F., Apovian, C.M., Livingston, E.H., Brolin, R., Sarwer, D.B., Anderson, W.A. & Dixon, J. (2008) Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract, 14(3), 318-36.
Publisher
11.Nguyen, N.T., Cronan, M., Braley, S., Rivers, R. & Wolfe, B.M. (2003) Duplex ultrasound assessment of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc, 17(2), 285-90.
Publisher
12.PDR 2013 – Physicians’ Desk Reference®: Product Labeling Lovenox – enoxaparin sodium injection: http://www.pdr.net/drug-summary/lovenox?druglabelid=521&id=1210. Last accessed Nov 18, 2013.
13.Podnos, Y.D., Jimenez, J.C., Wilson, S.E., Stevens, C.M. & Nguyen, N.T. (2003) Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg, 138(9), 957-61.
Publisher – Google Scholar
14.Prystowsky, J.B., Morasch, M.D., Eskandari, M.K., Hungness, E.S. & Nagle, A.P. (2005) Prospective analysis of the incidence of deep venous thrombosis in bariatric surgery patients. Surgery, 138(4), 759-63; discussion 763-5.
Publisher
15.Rocha, A.T., de Vasconcellos, A.G., da Luz Neto, E.R., Araujo, D.M., Alves, E.S. & Lopes, A.A. (2006) Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg, 16(12), 1645-55.
Publisher
16.Rondina, M.T., Wheeler, M., Rodgers, G.M., Draper, L. & Pendleton, R.C. (2010) Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-Ill patients. Thromb Res, 125(3), 220-3.
Publisher
17.Rowan, B.O., Kuhl, D.A., Lee, M.D., Tichansky, D.S. & Madan, A.K. (2008) Anti-Xa levels in bariatric surgery patients receiving prophylactic enoxaparin. Obes Surg, 18(2), 162-6.
Publisher
18.Rutherford, E.J., Schooler, W.G., Sredzienski, E., Abrams, J.E. & Skeete, D.A. (2005) Optimal dose of enoxaparin in critically ill trauma and surgical patients. J Trauma, 58(6), 1167-70.
Publisher
19.Sapala, J.A., Wood, M.H., Schuhknecht, M.P. & Sapala, M.A. (2003) Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg, 13(6), 819-25.
Publisher
20.Schauer, P.R. & Sirinek, K.R. (1995) The laparoscopic approach reduces the endocrine response to elective cholecystectomy. Am Surg, 61(2), 106-11.
21.Simone, E.P., Madan, A.K., Tichansky, D.S., Kuhl, D.A. & Lee, M.D. (2008) Comparison of two low-molecular-weight heparin dosing regimens for patients undergoing laparoscopic bariatric surgery. Surg Endosc, 22(11), 2392-5.
Publisher
22.Westling, A., Bergqvist, D., Bostrom, A., Karacagil, S. & Gustavsson, S. (2002) Incidence of deep venous thrombosis in patients undergoing obesity surgery. World J Surg, 26(4), 470-3.
Publisher – Google Scholar