Introduction
Overweight and obesity are the leading causes for the development of adverse metabolic effects, including non-insulin dependent diabetes mellitus, dyslipidemia, and cardiovascular disease (Bays, 2012). It has been suggested that dysregulated secretion of various factors from adipocytes and hepatocytes contributes to these metabolic effects (Galic, Oakhill, & Steinberd, 2010).
Retinol-binding protein 4 (RBP4) is a transport protein for retinoid (vitamin A) in the blood. It is secreted primarily from the liver and adipose tissue (Kotnik, Fischer-Posovszky, & Wabitsch, 2011). RBP4 has been proposed as an adipokine involved in the pathogenesis of insulin resistance (Yang et al., 2005; Graham et al., 2006). Adipose tissue RBP4 mRNA expression and circulating plasma levels have been shown to be increased in several mouse models of obesity and insulin resistance , and deleting the RBP4 gene in mice has been shown to increase insulin sensitivity (Yang et al., 2005). The link between RBP4, obesity, and insulin resistance in humans is less clear. While several clinical studies in adults confirmed this association (Graham et al., 2006; Cho et al.,2006), other studies have found no link between RBP4 and obesity and/or insulin resistance (Janke et al.,2006 ; Takashima et al., 2006).
Obese men have been shown to have elevated circulating estrogen levels predominantly derived from increased aromatase activity, which irreversibly converts testosterone (T) to estradiol (E2) resulting in decreased T and elevated E2 serum levels (Bulun et al., 2003). In cell culture experiments, it was found that estradiol directly increases the RBP4 mRNA expression, protein levels, and secretion of RBP4 into the culture media (Tan et al., 2007). Visceral adiposity and insulin resistance are known to be associated with lower T levels (Jones, 2010; Allan et al., 2008). Several studies indicate that visceral adipose tissue accumulation was the stronger predictor of RBP4 levels (Kloting et al., 2007; Jia et al., 2007).
Thus, the aim of the present study is to determine RBP4 serum levels and evaluate its relationship with T, E2, and E2/T ratio and insulin resistance in overweight and obese Egyptian men.
Subjects and Methods
Subjects:
The study included sixty five adult males. They were selected from working staff in Medical Research Institute and their relatives. Their age ranged from 38 to 60 years with a mean of (43.88±5.52) years. All participants gave their approval to participate in the study and a written consent was obtained from each subject. The Ethics Committee of the Medical Research Institute, Alexandria University; approved the study protocol, and all the experimental procedures were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Detailed history was taken from all subjects .They were apparently healthy upon complete physical examination, and were not using any medications or supplements.
According to the WHO criteria for definition of overweight and obesity on the basis of body mass index(BMI) (WHO,1998), subjects included in the study were divided into normal weight group (n20) with BMI (18.5-24.9), overweight group (n20) with BMI (25-29.9) and obese group (n25) with BMI (30-39.9).
Methods:
A- Anthropometric measurements: made by a single, well trained examiner on participant wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by square of the height in meters (Kg/m2). In addition, waist and hip circumferences were measured at the mid distance between iliac crest and last rib margin at the level of symphysis pubis and the maximum protrusion of the buttocks respectively with a soft tape while subjects were in the standing position. The waist to hip ratio (WHR) was then calculated.
b- Laboratory investigations which included: Fasting and 2hrs serum glucose concentrations after ingestion of 75 g glucose solution. Subjects with impaired glucose tolerance (fasting glucose > 110mg/dl and/or 2hrs OGTT glucose >140mg/dl) were excluded from the present work. Complete lipid profile (TC, HDL-C, LDL-C and TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma glutamyl transpeptidase (GGT) activities as well as urea and creatinine concentrations. Analysis was performed on the auto analyzer Konelab 30i system using reconstituted freeze dried forms of multianalyte calibrators for the serum samples. Serum levels of fasting insulin, total testosterone (T) and 17β estradiol levels (E2) using an IMMULITE 2000 analyzer , E2/T ratio was then calculated. Serum RBP4 concentration by Enzyme-Linked Immunosorbant assay using a commercially available kit Immunodiagnostik AG, Stubenwold-Alke 8a D64625 Benshemin according to manufacturer’s instructions.
Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR). The equa¬tion used was: HOMA-IR = (fasting insulin [µIU/ml] × fasting glucose [mmol/l])/22.5, while insulin sensitivity was estimated using the quantitative insulin sensitivity check index (QUICKI) using the following equation: QUICKI=1/ [log (fasting insulin (µIU/mL) + log (fasting glucose (mg/dL)].
Statistical Analysis
Statistical analysis was performed using SPSS software version 20. Normality was assessed by the Kolmogorov-Smirnov test. Data were described as (mean±SD) or median (range). For a comparison between the groups, ANOVA and Kruskal Wallis test were applied to normally and non-normally distributed parameters, respectively. When there was a significant effect, Post hoc test (Tuky) and Mann-Whitney U tests were performed for pair wise comparison. Pearson’s and Spearman Rank correlation coefficients were calculated to evaluate the relationship between serum RBP4 levels and clinical and metabolic variables. In order to determine which contributor was most strongly associated with RBP4 in the studied subjects, a multiple linear regression test was performed. P value < 0.05 was con¬sidered as statistically significant.
Results
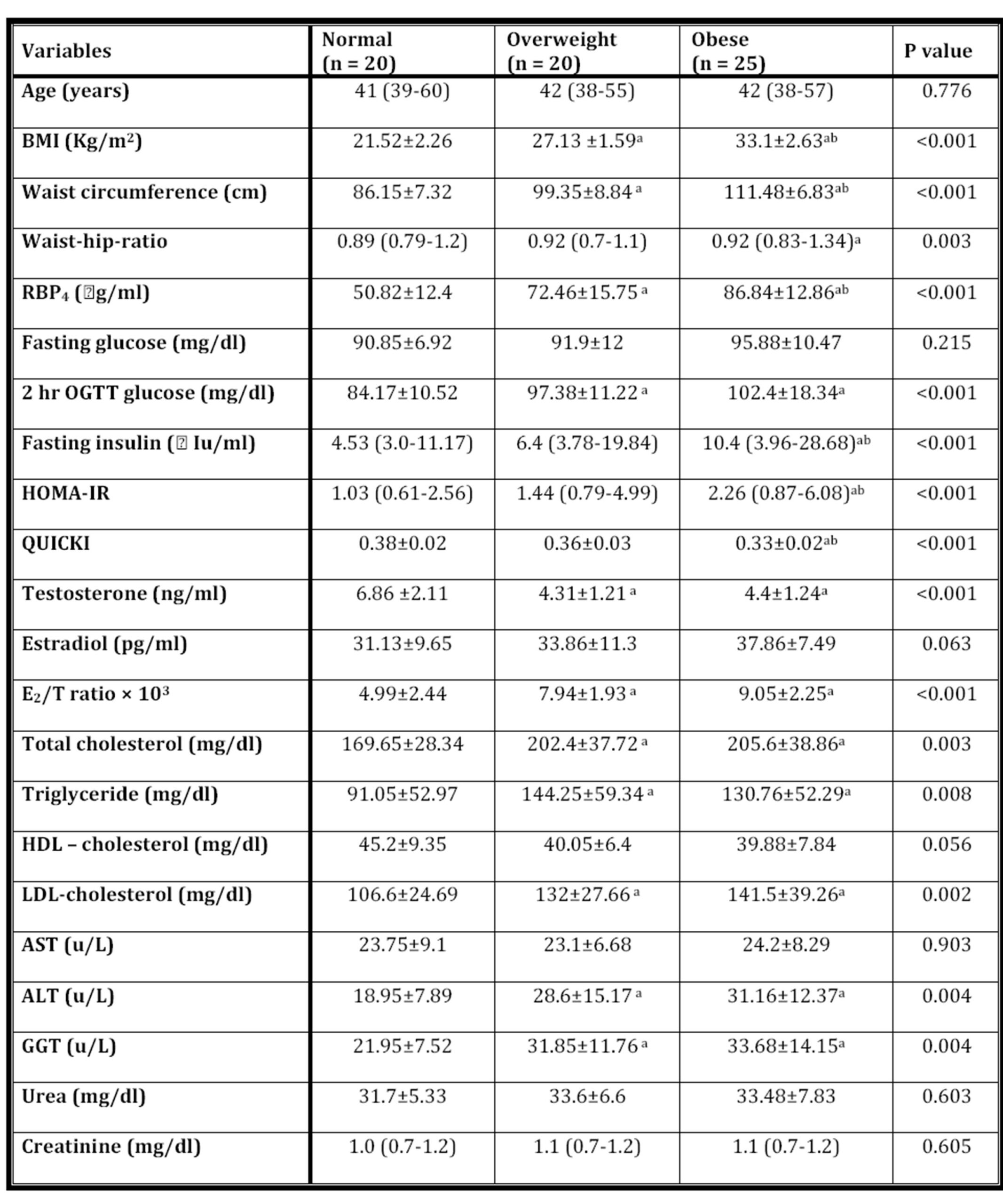
The clinical and metabolic variables of normal (n=20), overweight (n=20) and obese (n=25) groups were shown in table 1. Obese group had significantly lower QUICKI and higher BMI, waist circumference, RBP4, fasting insulin and HOMA-IR than those of overweight and normal groups. Total testosterone in obese group was significantly lower than those of normal group, while WHR, 2hrs oral glucose tolerance test (OGTT) glucose, E2/T ratio, total cholesterol, triglycerides, LDL-C, ALT and GGT were significantly higher. As regard overweight group, body mass index, waist circumference, RBP4, 2hrs (OGTT) glucose, E2/T ratio, total cholesterol, triglycerides, LDL-C, ALT, and GGT were significantly higher, while T was significantly lower as compared with normal weight group. In addition, age, fasting glucose, estradiol, HDL-C, AST, urea and creatinine didn’t show any statistically significant difference between the studied groups.
Table 1: the clinical and metabolic variables of normal, overweight and obese groups
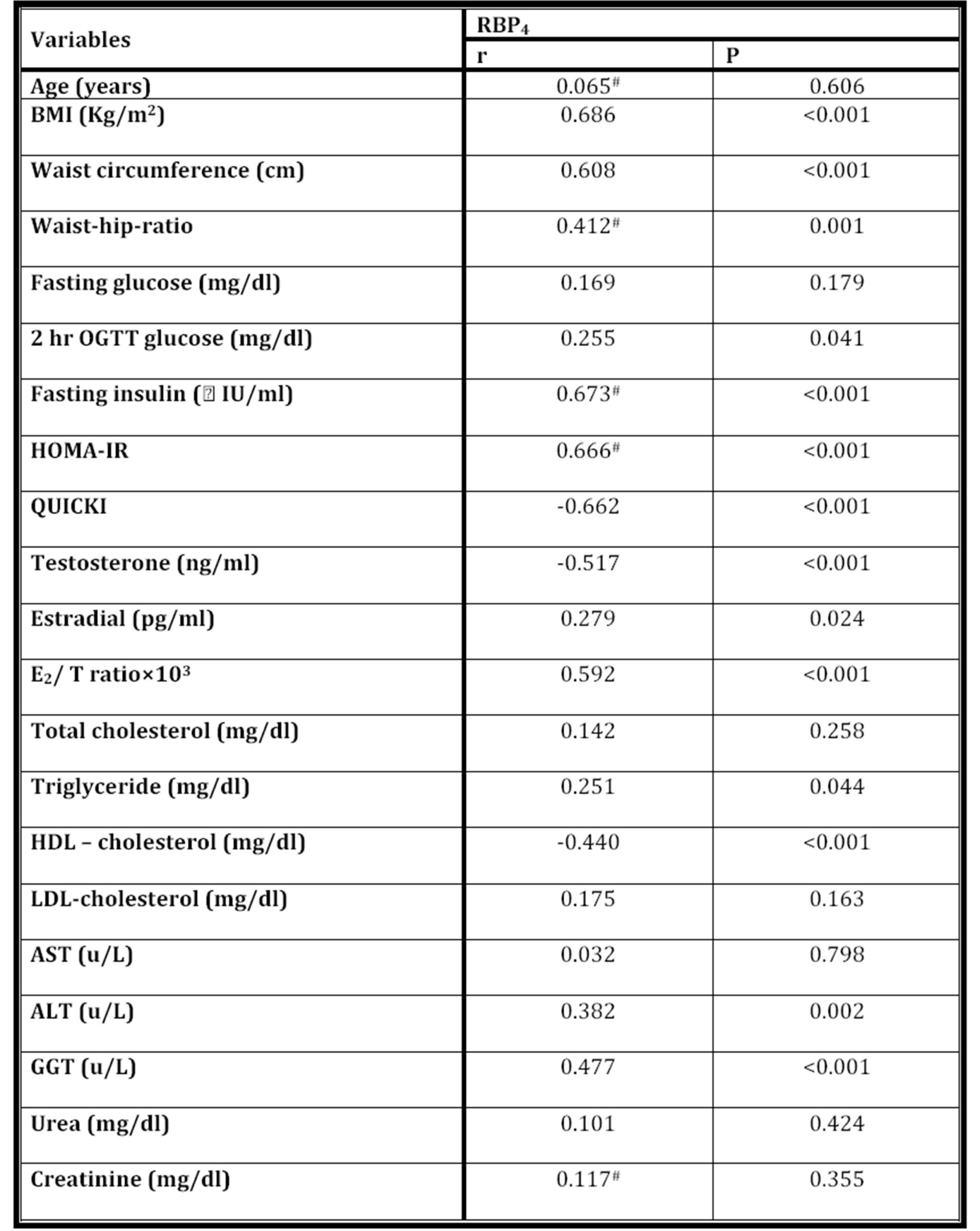
The correlations between serum RBP4 levels and other clinical and metabolic variables for all subjects included in the study were considered for analysis as shown in table 2. It was found that RBP4 serum level was positively correlated with BMI, waist circumference, WHR, 2hrs (OGTT) glucose, fasting insulin, HOMA-IR, E2, E2/T ratio, triglycerides, ALT and GGT, and it was negatively correlated with QUICKI, T and HDL-cholesterol.
Table 2: The correlation between RBP4 levels and various parameters (N = 65)
Serum RBP4 level was positively correlated with HOMA-IR in overweight (rs= 0.531 p=0.016) and obese (rs =0.423 p=0.035) groups. No significant correlation was present in normal weight group (Fig 1a,b,c).
RBP4 serum levels were negatively correlated with testosterone levels in the three studied groups, but only significant negative correlation was detected in the obese group (r= -0.5 p= 0.011) (Fig 2a,b,c).
In addition, RBP4 serum levels correlated positively with E2/T ratio which were statistically significant in the obese group only (r= 0.407 p=0.044) (Fig 3a,b,c).
In the multiple linear regression analysis, all predictors which significantly correlated with RBP4 by univariate analysis were tested for multicolinearity. The predictors which caused multicolinearity problems were omitted. The model included RBP4 (as a dependent variable) and waist circumference, 2hrs (OGTT) glucose, E2/T ratio, TG, HDL-C, and ALT (as independent variables). It was found that E2/T ratio was positive while, HDL-cholesterol was negative predictor of RBP4 serum levels (R2=0.551, F= 11.864, P<0.001) (Table 3).
Table (3): Multiple regression analysis with RBP4 as dependent variable ( R2= 0.551 p>0.001)
Discussion
Glucose transporter type 4 (GLUT4) is the principal insulin stimulated glucose transporter protein that mediates glucose uptake in adipose tissue and skeletal muscle, and it is thus a key regulator of glucose homeostasis (Huang & Czech, 2007). Reduced expression of GLUT4 in adipocytes is an early pathological feature of insulin resistance which precedes glucose intolerance (Smith, 2002).The onset of this insulin resistant condition is intimately associated with weight gain, as the target tissues such as muscle and liver fail to adjust glucose metabolism appropriately in response to insulin (Abel, Peroni & Kim, 2001).
Retinol binding protein 4 attracted a considerable attention as an adipokine that provides a possible link between expression of adipose GLUT4 in adipocytes and insulin resistance. Many studies showed that a decreased GLUT4 expression by adipose tissue causes an increased RBP4 synthesis and secretion, suggesting that RBP4 might be the link between adipose tissue and insulin resistance induction in the muscle and liver (Abel, Peroni & Kim, 2001; Yang et al., 2005). In the present study, serum RBP4 levels were elevated in overweight and obese subjects, and correlated positively with BMI. These results are in accordance with previous studies (Yang et al., 2005; Graham et al., 2006).
In the present study, serum RBP4 levels were elevated in overweight and obese subjects and correlated positively with BMI. These results are in accordance with previous studies (Yang et al., 2005; Graham et al., 2006).
Dysfunctions of both, the liver and kidneys, are known to influence RBP4 homeostasis. Chronic kidney diseases and chronic liver diseases interfere with RBP4 metabolism through their actions on RBP4 synthesis and catabolism (Tönjes, Blüher & Stumvoll, 2010; Henze, Frey, & Raila, 2008). That’s why, subjects with impaired liver or kidney functions were excluded from the present study.
Several studies demonstrated that changes in RBP4 have systemic effects on insulin sensitivity and glucose homeostasis in humans (Yang et al., 2005; Graham et al., 2006). It is believed that RBP4 affects insulin sensitivity by down regulating the activities of phosphoinositide 3-kinase and insulin stimulated tyrosine phosphorylation of insulin receptor substrate-1 in muscle, which are the key steps in glucose metabolism (Yang et al., 2005). Moreover, RBP-4 increases hepatic glucose production by up regulating the expression of phosphoenolpyruvate carboxykinase (Yang et al., 2005), a master regulation of hepatic glucose production and a major downstream target of insulin signaling.
In the present study, homeostatic model assessment of insulin resistance (HOMA-IR) was significantly higher, while insulin sensitivity index (QUICKI) was significantly lower in obese group as compared to both overweight and normal groups. Moreover, serum RBP4 correlated positively with HOMA-IR and negatively with QUICKI in both overweight and obese groups. These findings are in agreement with the previous studies (Yang et al., 2005; Graham et al., 2006; Cho et al., 2006).
The earliest abnormality observed in insulin resistance is a decrease in insulin induced glucose uptake in skeletal muscle and adipose tissue, as well as dyslipidemia (Saltiel, A.R. & Kahn, C.R., 2001). In the present study, 2hr (OGTT) glucose and lipid profile (total cholesterol, T.G, and LDL-C) were all significantly higher in overweight and obese groups as compared to normal group. Moreover, RBP4 serum levels showed significant positive correlations with 2hrs (OGTT) glucose and T.G, and negative correlation with HDL-C.
The elevated 2hrs (OGTT) glucose, could be explained by the findings of Broch et al.,2007 , who stated that RBP4 may impair β-cell function in human subjects, as RBP4 circulates in serum forming a complex with transthyretin, which constitutes a functional component in pancreatic β-cell stimulus secretion coupling. Thus, it is possible that an increased serum RBP4 prevents the transthyretin from exerting its β-cell stimulus secretion effects. In addition, RBP4 might have a direct role in the progression of lipogenesis, as it was found that RBP4 increased the expression of the gene encoding fatty acid synthase in adipose tissue (Berndt et al., 2007).
Multiple studies revealed that the obese men had higher levels of circulating estradiol or elevated estradiol/testosterone ratios (Schneider et al., 1979; Jensen et al., 2004; Fejes et al.2006). The overall rate of aromatization of testosterone to estradiol increases with age and fat mass (Vermeulen et al., 2002). This alteration in the estradiol/testosterone ratio may lead to further abdominal fat deposition and greater degree of testosterone deficiency.
In the present study, testosterone level was significantly lower in overweight and obese groups as compared with normal group. Moreover, RBP4 levels correlated negatively with T in the obese group. These findings could be explained by the observation of Allan et al., 2008, who demonstrated that changes in visceral fat appeared to be a function of changes in serum T levels, and prospective studies have confirmed that lower endogenous androgens predict central adiposity in men (Khaw & Barrett-Connor, 1992). In a study done by Kloting et al., 2007, RBP4 expression was observed to be higher in visceral adipose tissue than subcutaneous adipose tissue. In addition, association with plasma RBP4 was stronger for visceral fat RBP4 expression than for subcutaneous adipose tissue RBP4 expression.
In the current study, RBP4 correlated positively with waist circumference and WHR both of which are closely correlated with visceral fat content in clinical studies ( Weits et al., 1998).
Although mean estradiol serum level was higher in both overweight and obese groups as compared with normal group, no statistically significant difference was detected between the 3 studied groups (p= 0.06). As regard E2/T ratio, it showed a statistically significant increase in overweight and obese groups as compared to normal group. Moreover, in the obese group RBP4 correlate positively with E2/T ratio. In multiple linear regression analysis E2/T ratio independently affected RBP4 levels.
Small increases in androgens aromatization can result in substantial changes in the circulating estrogens levels, these being biologically active at much lower concentrations than androgens (Akingbemi BT. 2005). Data suggest that in the physiological range E2 is beneficial for insulin sensitivity whereas hypo or hyperestrogenism is related to insulin resistance (Livingstone & Collison, 2002). An up regulation of RBP4 mRNA expression in human adipose tissue explants by 17 β estradiol was demonstrated (Tan et al., 2007).
The increased estradiol concentration in obese men (Schneider et al., 1979) influences both estrogen receptors (ER). Estrogen receptors alpha and beta seem to have opposing effects on the expression of GLUT4 transporters. ER alpha was shown to induce GLUT4 expression, whereas ER beta seems to inhibit GLUT4 expression in skeletal muscle and white adipose tissue (Barros et al., 2006; Barros et al., 2009). Nilsson et al.,2007, showed that ER alpha is diminished in adipose tissue from obese compared with normal subjects, suggesting increased ER beta activity which adversely affects GLUT4 expression (Barros et al., 2006; Barros et al., 2009) and increases RBP4 expression resulting in insulin resistance (Yang et al.,2005; Graham et al., 2006). This was clearly demonstrated in a case report of a male patient with ER alpha loss of function and glucose intolerance associated with insulin resistance (Smith, Boyd & Frank 1994). This was believed to be a consequence of unopposed ER beta expression due to a loss of ER alpha activity.
Even if the adipose tissue might be a less important source of circulating RBP4 in human as has been urged by Janke et al., 2006, and that hepatocytes are considered as the major source of RBP4 under normal conditions (Tsutsumi et al., 1992), direct effect of T and or E2 on RBP4 expression from liver or adipose tissue could not be excluded, as normal liver and adipocytes express androgen as well as estrogen receptors alpha and beta (Kahn et al., 1989; Nilsson et al.,2001; Dieudonne et al.,1998; Pedersen et al., 2001). Both androgen receptors & ER belong to the family of nuclear receptors that act as transcription factors that regulate the expression of several genes (Boonyaratanakornkit & Edwards, 2007). This is in coincidence with Lin et al., 2008, who noticed reduced insulin sensitivity in hepatic androgen receptors knockout male mice, without impaired development of genital organs and subsequent hypogonadism. This decrease in insulin sensitivity was associated with reduced phosphoinositide 3-kinase activity and increased the phosphoenol pyruvate carboxykinase expression in the liver, which is the same mechanism by which RBP4 induces insulin resistance (Yang et al., 2005).
In conclusion, serum RBP4 levels were elevated in overweight and obese men as compared with normal weight subjects and were associated with increased E2/T ratio and HOMA-IR in obese men. In addition, direct effect of T and or E2 on RBP4 expression from liver or adipose tissue could not be excluded. Finally, the disturbance in E2/T ratio seems to affect RBP4 serum levels and insulin sensitivity in obese men. Future researches are needed to determine how aromatase inhibitors can be effectively applied to correct these complex metabolic abnormalities without the generation of other adverse effects.
References
1. Abel, E.D., Peroni, O., Kim, J.K., et al. (2001). Adipose- selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature , 409:729-733.
Publisher – Google Scholar
2. Akingbemi BT. Estrogen regulation of testicular function. Reprod Biol Endocrinol. 2005;27: 3—51.
Google Scholar
3. Allan, C.A., Strauss, B.J., Burger, H.G., et al. (2008).Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in non obese aging men. J Clin Endocrinol Metab, 93:139-146.
Publisher – Google Scholar
4. Barros, R.P., Gabbi, C., Morani, A., et al. (2009). Participation of ER alpha and ER beta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab , 297: E124-E 33.
Google Scholar
5. Barros, R.P., Machado, U..F, Warner, M.& Gustafsson, J.A. (2006). Muscle GLUT4 regulation by estrogen receptors ER beta and ER alpha. Proc. Natl. Acad. Sci. USA , 103: 1605-1608.
Publisher – Google Scholar
6. Bays, H. E. (2012). Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating “sick fat” through improving fat function with antidiabetes therapies. Am J Cardio, 6; 110 :4B-12B.
Publisher – Google Scholar
7. Berndt, J., Kovacs, P., Ruschke, K., et al. (2007). Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia , 50:1472-1480.
Publisher – Google Scholar
8. Boonyaratanakornkit, V. & Edwards, D.P.(2007). Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med , 25:139-153.
Publisher – Google Scholar
9. Broch, M., Vendrell, J.W., Ricart, W.C. , et al.(2007). Circulating retinol-binding protein 4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes care, 30:1802-1806.
Publisher – Google Scholar
10. Bulun, S.E., Sebastian, S., Takayama, K., et al. (2003). The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol, 86: 219-224.
Publisher – Google Scholar
11. Cho, Y.M., Youn, B.S., Lee, H., et al. (2006). Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care, 29:2457-2461.
Publisher – Google Scholar
12. Dieudonne, M.N., Pecquery, R., Boumediene, A., et al. (1998). Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol , 274 : C1645- C1652.
Google Scholar
13. Fejes, I., Kolosza, S., Zavackcski, Z., et al.(2006). Effect of body weight on testosterone/estradiol ratio in olgozoospermic patients. Arch Androl , 52:97-102.
Publisher – Google Scholar
14. Galic, S., Oakhill, J.S., & Steinberg, G.R. (2010). Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology, 316: 129-139.
Publisher – Google Scholar
15. Graham, T.E., Yang, Q., Bluher, M., et al. (2006). Retinol binding protein 4 and insulin resistance in lean and obese subjects and subjects with type 2 diabetes. N Engl J Med, 354: 2552-2563.
Publisher – Google Scholar
16. Henze, A., Frey, S.K., & Raila, J. (2008). Evidence that kidney function but not type 2 diabetes determines retinol-binding protein 4 serum levels. Diabetes, 57:3323-3326.
Publisher – Google Scholar
17. Huang, S., & Czech, M., P. (2007). The GLUT4 glucose transporter. Cell Metab ,5:237-252.
Google Scholar
18. Janke, J., Engeli, S., Boschmann, M., et al. (2006). Retinol-binding protein 4 in human obesity. Diabetes, 55: 2805-2810.
Publisher – Google Scholar
19. Jensen, T.K., Andersson, A.M., Jorgensen, N., et al.(2004). Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril , 82:863-870.
Publisher – Google Scholar
20. Jia, W., Wu, H., Bao, Y., et al. (2007). Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab, 92: 3224-3229.
Publisher – Google Scholar
21. Jones, T.H. (2010). Testosterone deficiency: a risk factor for cardiovascular disease. Trends Endocrinol Metab, 21: 496-503.
Publisher – Google Scholar
22. Kahn, D., Zeng, Q., Kajani, M., et al. (1989).The effect of different types of hepatic injury on the estrogen and androgen receptor activity of liver. J Invest Surg, 2: 125-133.
Publisher – Google Scholar
23. Khaw, K.T. & Barrett-Connor, E. (1992) Lower endogenous androgens predict central adiposity in men. Ann. Epidemiol , 2: 675- 682.
Publisher – Google Scholar
24. Kloting, N., Graham, T.E., Berndt, J., et al. (2007). Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metabolism, 6: 79-87.
Publisher – Google Scholar
25. Kotnik, P., Fischer-Posovszky, P., &Wabitsch, M. (2011). RBP4: a controversial adipokine. Eur J Endocrinol, 165:703-711.
Publisher – Google Scholar
26. Lin, H.Y.,Yu, I.C.,Wang, R.S.,et al. (2008). Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatol , 47: 1924-1935.
Publisher – Google Scholar
27. Livingstone, C. & Collison, M. (2002). Sex steroids and insulin resistance. Clin. Sci, 102:151-166.
Publisher – Google Scholar
28. Nilsson, M., Dahlman, I., Rydén, M., et al.(2007). Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond) , 31: 900- 907.
Publisher – Google Scholar
29. Nilsson, S., Mäkelä, S., Treuter, E., et al. (2001). Mechanisms of Estrogen Action. Physiol Rev, 81:1535-1565.
Google Scholar
30. Pedersen, S.B., Bruun, J.M., Hube, F., et al.(2001). Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol , 182:27-37.
Publisher – Google Scholar
31. Saltiel, A.R. & Kahn, C.R. (2001). Insulin signaling and the regulation of glucose and lipid metabolism .Nature; 414: 799-806.
Publisher – Google Scholar
32. Schneider, G., Kirschne, M.A., Berkowitz, R. & Ertel, N.H.(1979). Increased estrogen production in obese men. J Clin Endocrinol Metab , 48:633-638.
Publisher – Google Scholar
33. Smith, E.P., Boyd, J. & Frank, G.R. (1994). Estrogen resistance caused by a mutation in the estrogen-receptor gene in man. N Engl J Med , 331:1056-1061.
Publisher – Google Scholar
34. Smith, U.(2002). Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance – is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord , 26:897-904.
Google Scholar
35. Takashima, N., Tomoike, H., & Iwai, N. (2006). Retinol-binding protein 4 and insulin resistance. N Engl J Med, 355:1394-1395.
Google Scholar
36. Tan, B.K., Chen, J., Lehnert, H., et al. (2007). Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab 92: 2764-2772.
Publisher – Google Scholar
37. Tönjes, A., Blüher, M. & Stumvoll, M. (2010). Retinol-binding protein 4 and new adipocytokines in nonalcoholic fatty liver disease. Curr Pharm Des, 16: 1921-1928.
Publisher – Google Scholar
38. Tsutsumi, C., Okuno, M., Tannous, L., et al.(1992). Retinoids and retinoid-binding protein expression in rat adipocytes. Journal of Biological Chemistry Liver , 267: 1805-1810.
Google Scholar
39. Vermeulen, A., Kaufma, J.M., Goemaere, S. & van Pottelberg, I. (2002). Estradiol in elderly men. Aging Male, 5: 98-102.
Publisher – Google Scholar
40. Weits, T., van der Beek, E.J., Wedel, M. &Ter Haar Romney, B.M. (1998). Computed tomography measurement of abdominal fat deposition in relation to anthropometry. Int J Obes , 12:215-17.
41. World Health Organization (1998): Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Geneva, WHO
42. Yang, Q., Graham, T.E., Mody, N., & Preitner, F., et al. (2005). Serum retinol binding protein contributes to insulin resistance in obesity and type 2 diabetes. Nature, 436:356 -362.
Publisher – Google Scholar