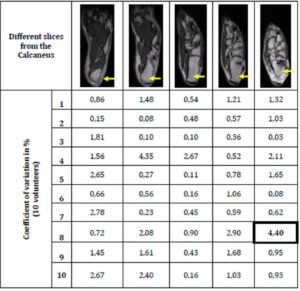
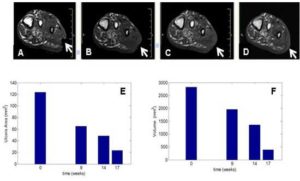
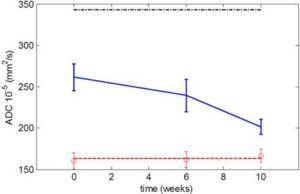
Acosta, J. B., Savigne, W., Valdez, C., Franco, N., Alba, J. S., del Rio, A., Lopez-Saura, P., Guillen, G., Lopez, E., Herrera, L. & Fernandez-Montequın, J. (2006). “Epidermal Growth Factor Intralesional Infiltrations Can Prevent Amputation in Patients with Advanced Diabetic Foot Wounds,” International Wound Journal, 3(3):232—239.
Publisher – Google Scholar
Andreassen, C. S., Jakobsen, J., Ringgaard, S., Ejskjaer, N. & Andersen, H. (2009). “Accelerated Atrophy of Lower Leg and Foot Muscles a Follow-Up Study of Long-Term Diabetic Polyneuropathy Using Magnetic Resonance Imaging (MRI),” Diabetologia, 52 (1):1182-1191.
Publisher – Google Scholar
Freund, W., Weber, F., Billich, C. & Schuetz, U. H. (2012). “The Foot in Multistage Ultra-Marathon Runners: Experience in a Cohort Study of 22 Participants of the Trans Europe Footrace Project with Mobile MRI,” BMJ Open, 2:e001118.
Publisher – Google Scholar
Frykberg, R. G., Zgonis, T., Armstrong, D. G., Driver, V. R., Giurini, J. M., Kravitz, S. R., Landsman, A. S., Lavery, L. A., Christopher Moore, J., Schuberth, J. M., Wukich, D. K., Andersen, C. & Vanore, J. V. (2006). “Diabetic Foot Disorders: A Clinical Practice Guideline,” Journal of Foot and Ankle Surgery, 45 (5): S1-S66.
Publisher – Google Scholar
Johnson, P. W., Collins, M. S. & Wenger, D. E. (2009). “Diagnostic Utility of T1-Weighted MRI Characteristics in Evaluation of Osteomyelitis of the Foot,” American Journal of Roentgenology, 192 (1):96-100.
Publisher – Google Scholar
Khoo, M. M. Y., Tyler, P. A., Saifuddin, A. & Padhani, A. R. (2011). “Diffusion-Weighted Imaging (DWI) in Musculoskeletal MRI: A Critical Review,” Skeletal Radiology, 40 (6):665—681.
Publisher – Google Scholar
López-Saura, P. A., Yera-Alos, I. B., Valenzuela-Silva, C., González-Díaz, O., del Río-Martín A., Berlanga- Acosta, J., Fernández-Montequín, J. I., Acevedo-Castro, B., López-Mola, E. & Herrera-Martínez, L. (2013). “Medical Practice Confirms Clinical Trial Results of the Use of Intralesional Human Recombinant Epidermal Growth Factor in Advanced Diabetic Foot Ulcers,” Advances in Pharmacoepidemiology & Drug Safety; 2 (2):1000128.
Publisher – Google Scholar
Mohan, V. (2013). “Has the Time Come for Routine Imaging Studies in Diabetics?,” Diabetes Technology and Therapeutics, 15(5): 349.
Publisher – Google Scholar
Sanverdi, S. E., Ergen, F. B. & Oznur A. (2012). “Current Challenges in Imaging of Thediabetic Foot,” Diabetic foot & Ankle, 3.
Publisher – Google Scholar
Schmid, M. R., Hodler, J., Vienne, P., Binkert, C. A. & Zanetti, M. (2002). “Bone Marrow Abnormalities of Foot and Ankle: STIR versus T1-Weighted Contrast Enhanced Fat-Suppressed Spin-Echo MR Imaging,” Radiology, 224 (2):463-469.
Publisher – Google Scholar
Suzuki, E., Kashiwagi, A., Hidaka, H., Maegawa, H., Nishio, Y., Kojima, H., Haneda, M., Yasuda, H., Morikawa, S., Inubushi, T. & Kikkawa, R. (2000). “1H- and 31P-Magnetic Resonance Spectroscopy and Imaging as a New Diagnostic Tool to Evaluate Neuropathic Foot Ulcers in Type II Diabetic Patients,” Diabetologia, 43 (2):165-172.
Publisher – Google Scholar
Vartanians, V. M., Karchmer, A. W., Giurini, J. M. & Rosenthal, D. I. (2009). “Is there a Role for Imaging in the Management of Patients with Diabetic Foot?,” Skeletal Radiology, 38 (7):633-636.
Publisher – Google Scholar