Introduction
Stroke is one of the leading causes of death, and is the largest cause of disability in developed countries (Donnan et al., 2008). Thrombolysis, using recombinant tissue plasminogen activator (tPA), is the most effective hyper-acute (within 24 hours) intervention for acute ischaemic stroke (AIS) (the initial stage at onset of symptoms due to thromboembolic occlusion which last for at least one hour) (NSF, 2010). It has been shown that, compared with patients given placebo, patients treated with tPA within 3 hours of stroke onset are at least 30% more likely to have minimal or no disability at three months (NINDS, 1995). Despite the overwhelming evidence that supports the safe and effective use of thrombolysis in the treatment of acute ischaemic stroke, and the recommendations endorsed by national and international stroke management guidelines (NSF, 2010), only a small proportion of stroke patients currently receive thrombolytic therapy (NSF, 2010, Adams et al., 2007, NSF 2011, and Rudd, 2011). It is hypothesised that treatment with thrombolysis in clinical practice is not in accordance with the accepted criteria from randomised studies and stroke management guidelines (NSF, 2010 and Adams et al., 2007), with potentially eligible patients missing out on thrombolytic therapy.
Aims
The aim of this study was to audit the utilization of thrombolysis in local hospital practice, and specifically determine: the proportion of patients potentially thrombolysis-eligible presenting to hospital; the rate of thrombolysis utilization among these patients; and factors associated with the utilization of thrombolysis.
Methods
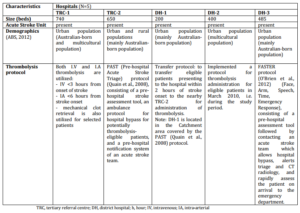
A retrospective, cross-sectional audit was conducted in five metropolitan hospitals in New South Wales (NSW), Australia; the hospitals consisted of two tertiary referral centres (TRC) and three district hospitals (DH) (Table 1). This sampling strategy aimed to capture a sample of the different types of hospitals offering stroke services to an Australian population. Patients admitted during the 12-month time period from July 2009 to July 2010, were initially identified from the medical records databases of each hospital via the International Classification of Diseases, tenth revision codes for acute ischaemic stroke (code I63.x, I64)( Kokotailo et al, 2005). A systematic sampling technique (i.e. starting at the first record and then proceeding with the selection of every nth record from a patient list ordered by admission dates) was employed when needed to select records for detailed review. This sampling technique was employed to enable the capture of admissions spread throughout the 12-month study period and to account for any seasonal variability. A sample size of at least 400 patients (>80 patients per hospital) was sought on the basis of a 20% estimate for the proportion of thrombolysis “eligible” patients (Quain et al., 2008), allowing for a precision of 4% and a confidence interval of 95%.
Table 1: Hospital Characteristics
A purpose-designed data collection form, modeled on one used in a previous audit study (Bajorek et al., 2002), was used to extract key information including patient demographics, medical history, medication history, admission and discharge data. Critical time points, including time of stroke onset, time of arrival, time of neuro-imaging, and time of thrombolysis administration, were also recorded. Time of stroke onset was defined as the time at which the patient or a bystander first noticed any neurological symptoms. For patients who were found with the symptoms, or in whom symptoms were first noticed after waking from sleep, the stroke onset time was recorded as the time the patient was last known to be symptom-free.
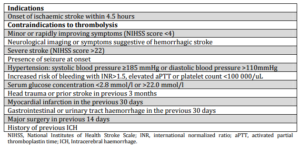
Patients presenting with an acute ischaemic stroke who arrived within the recommended time-window for the administration of thrombolysis (4.5 hours) and who did not have any documented contraindications to treatment (Table 2, as per the Clinical Guidelines for Stroke Management 2010, National Stroke Foundation, Australia) (NSF, 2010) were considered “potentially eligible” for thrombolysis. The appropriateness of treatment was determined according to the published evidence-based clinical guidelines (NSF, 2010 and Adams et al., 2007).
Table 2: Patient Selection Criteria for Thrombolysis
Data Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 19 (SPSS). The independent samples chi-squared test was used to compare categorical variables and for continuous variables we used either the Student’s t-test (for normally distributed variables) or the Mann—Whitney U test (for variables with skewed distributions). The following independent variables were tested: patient age, gender, stroke risk factors, previous history of stroke or TIA, pre-morbid degree of disability (modified Rankin Scale score) (Van Swieten et al., 1988), weekend admission, mode of hospital arrival, blood pressure on admission, serum glucose level on admission, stroke severity, onset-to-arrival time, onset-to-ambulance contact time, ambulance contact-to-arrival time and arrival-to-neuroimaging time. A P-value of <0.05 was considered statistically significant in all analyses.
Ethics approval was granted by the human research ethics committees of Northern Sydney Local Health District, Western Sydney Local Health District and Hunter New England Health District.
Results
Patient Characteristics
During the 12-month audit period, a total of 2,261 patients were admitted with acute stroke, of whom 55.8% (1,261 patients) presented with AIS. A sample of 521 medical records of AIS patients was reviewed and the distribution of cases by hospital is shown in Figure 1. Similar numbers of records were reviewed from tertiary referral centres (n=250) and district hospitals (n=271). Overall, the sample had similar proportions of males and females. The median age of all patients was 77.3 (Inter-quartile range — [IQR] 66.9-84.5) years and the majority were over 65 years of age. As expected and typical of the AIS patient population (NSF 2009) the most commonly reported co-morbid conditions were hypertension, hypercholesterolemia, previous stroke or transient ischaemic attack (TIA) and atrial fibrillation (AF) (Table 3), with the overall demographic characteristics very similar to the Australian national stroke audit data (NSF 2009).
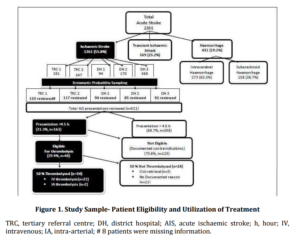
Table 3: Demographic Characteristics of all Acute Ischaemic Stroke Patients (n=521)

Patient Presentation
In regard to patient presentation to hospital, 80.0% presented via ambulance (ranging from 72.0% at DH-2 and DH-3 to 85.0% at DH-1 and TRC-2). Among all AIS patients, 83.0% presented with an elevated mean arterial blood pressure (BP) greater than 100 mmHg on admission (7.3% systolic BP >185 and 3.9% diastolic BP >100, and 30.0% had an elevated serum glucose level (>8 mmol/l). The length of stay ranged from 1 to 49 days (median 7.0 days, IQR 4.0-11.0) with 78.0% of all patients admitted to a dedicated acute stroke unit.
Eligibility for Thrombolysis
Overall, almost one third of all AIS patients arrived within the time window for thrombolysis (4.5 hours). Among these patients, 48 patients (30.0% of those arriving within the time window) were identified as being potentially eligible for thrombolysis according to guidelines (NSF, 2010) (Figure 1). Overall, 9.2% (95% CI, 6.7%-11.7%) of all AIS patients were eligible for thrombolysis, although the proportion of thrombolysis-eligible patients across individual hospitals ranged from 4.3% (95% CI, 0.22%-8.38%) (DH-1) to 12.8% (95% CI, 6.7%-18.9%) (TRC-2).
Utilization of Thrombolysis
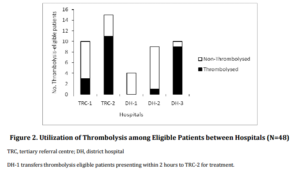
The National Institutes of Health Stroke Scale (NIHSS) score on admission was documented in 41.5% of AIS patients. Documented evidence that thrombolysis was considered as a treatment option was identified in 28.4 % of AIS patients overall, and in only two-thirds (66.0%) of those deemed to be thrombolysis-eligible (irrespective of whether the patient received thrombolysis or not). However, this proportion varied between hospitals: 50.0% DH-1, 11.1% DH-2, 70.0% TRC-1, 80.0% TRC-2 and 100% at DH-3. It is worth noting here that DH-2 had started to implement a protocol for thrombolysis administration only in March 2010 (during the study period).
A different trend was seen in the rate of actual thrombolysis utilization among thrombolysis-eligible patients, with a significant difference in the utilization of thrombolysis between the different hospitals (P<0.01) (Figure 2). Overall, only half of the patients who were deemed eligible for thrombolysis actually received the treatment (Figure 1). The overall utilization of thrombolysis was 4.6% among all AIS patients and 14.7% among patients arriving within the time-window of 4.5 hours.
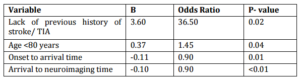
Four independent variables were identified as having an impact on the likelihood of thrombolysis. Age >80 years, lack of previous history of stroke or TIA, shorter onset-to-arrival time, and shorter arrival-to-neuroimaging time (P<0.05) (Table 4).
Table 4: Factors Associated with the Utilization of Thrombolysis among Eligible Patients
Discussion
The results of this study, which included over 500 patients admitted to a range of hospitals, confirm that thrombolysis is being underutilized in clinical practice, with some stroke patients who are considered potentially eligible for thrombolysis not being treated, which is consistent with the low utilization of thrombolysis recently reported in another Australian state (Leyden et al., 2011). This study also highlights the wide variation in the utilization rates between Australian hospitals even within the same region. This is supported by the fact that some Australian hospitals have reported thrombolysis rates of up to 20.0% (Kokotailo et al, 2005).
Approximately 1 in 10 AIS patients were found to be eligible for thrombolysis in this study, which is similar to the proportion (11.0%) previously reported for the conventional 3-hour time window in Australia (Gilligan et al., 2005). This is the first Australian study to report the proportion of AIS patients ‘potentially eligible’ for thrombolysis according to the ‘extended time-window for thrombolysis’ stroke management guidelines (NSF, 2010), irrespective of whether they received thrombolysis or not. The proportion of patients potentially eligible for thrombolysis may be an underestimate, given the difficulties in capturing all pertinent information from the medical records, but the fact that only approximately half of these patients received treatment supports the need to identify critical points for intervention to increase the scope of use. Large scale Australian thrombolysis implementation trials are currently underway including (TIPS — Thrombolysis ImPlementation Stroke trial) and (T3 Trial— Triage, Treatment and Transfer of Patients with stroke in Emergency Departments). International studies have found similar proportions of patients being eligible for thrombolysis, ranging from 7 to 16% (Rudd, 2011, Barber et al., 2001 and Katzan et al., 2004). Among patients presenting within the time window for thrombolysis, this study found that approximately 1 in 3 (30%) were eligible for thrombolysis, which is slightly lower than the proportion reported by the other studies – ranging from 46%-59% (Barber et al., 2001 and Katzan et al., 2004). The most important finding of this study is the suboptimal utilization of thrombolysis in ‘eligible’ patients, where only 1 in 2 eligible patients received the treatment. Other studies in the US and Canada have reported similar findings, with only 44% and 46% of eligible patients being treated respectively (Barber et al., 2001 and Katzan et al., 2004).
Among the thrombolysis-eligible patients in this study, three underwent mechanical clot removal rather than being administered thrombolysis. No RCTs (randomised controlled trials) have investigated this procedure for acute stroke but several observational studies have reported it to be beneficial (Stead et al., 2008). However, this procedure is not expected to be widely implemented across Australia due to the limited number of centres capable of providing this service, given the high associated costs and need for specialised interventional expertise.
Although older age (>80 years) is not an absolute contraindication for thrombolysis (NSF, 2010), this study indicates lower utilization of thrombolysis among these patients. The recently published IST-3 (third international stroke trial) which included 1617 patients older than 80 years treated up to 6 hours from stroke onset clearly demonstrated that thrombolysis utilization in elderly patients (>80 years) is both safe and effective (Sandercock et al., 2012). The IST-3 will certainly enhance the utilization of thrombolysis in elderly patients in the future.
An interesting finding of this study, which has not been reported before, is that patients with a history of prior stroke or TIA appear less likely to receive thrombolysis. None of the RCTs, national or international guidelines exclude patients from treatment based on a history of prior stroke or TIA. This may reflect difficult to measure factors such as the treating clinician’s perception of patient “frailty” or an interpretation of the baseline CT scan as “unfavourable to treat” for patients with previous stroke. Patient frailty has previously been recognized in the literature as a predictor of treatment with other cardiovascular therapies for stroke (Perera et al., 2009).
Similar to findings of a US study (Katzan et al., 2004), appropriate medical record documentation of ineligibility for thrombolysis is lacking in some Australian hospitals, specifically the documentation of stroke severity on admission using a validated stroke-specific tool (e.g. National Institutes of Health Stroke Scale and Scandinavian Stroke Scale) as recommended by the guidelines (Adams et al., 2007). Ideally this should be documented for every AIS patient, or at least for those presenting within the time window for thrombolysis.
In interpreting the study findings, it is important to note some limitations. Firstly, it was a retrospective study, relying on ICD-10 codes to identify patients with AIS. Secondly, patient eligibility for thrombolysis was based on information extracted from medical records, whereas there could have been other undocumented reasons rendering the patient ineligible for treatment. In addition, patients arriving at the end of the time window may be ineligible for treatment due to insufficient time for diagnostic imaging and treatment. Furthermore, we relied on documented stroke scale scores, such as the retrospective mRS scores, to assess patient’s pre-morbid degree of disability (Van Swieten et al., 1988). Lastly, only five metropolitan hospitals from a single state (NSW) were included in the study, and therefore may not be representative of other hospitals within the same state or other states across Australia. Furthermore, no rural hospitals were included in this study. However, in Australia less than 12% of stroke presentations are admitted to rural hospitals (NSF 2011).
In spite of these limitations, this study highlights that in actual clinical practice the utilization of thrombolysis is potentially suboptimal, also the variation in the utilization of thrombolysis shows that optimal utilization can be facilitated within the currently available resources in Australian hospitals provided that a coordinated multidisciplinary approach is adopted involving pre-hospital, emergency department, and acute stroke teams.
In conclusion, the prevalence of thrombolysis-eligible patients is apparently low (less than 10% of AIS patients), mainly due to delayed patient presentation. However, even when patients are eligible, thrombolysis is underutilized with up to 50% of patients not being treated according to the guidelines. To minimize the risk of disability from AIS, acute stroke care management needs to be standardized. More effort is needed in translating the models of care in successful centres to other sites, to improve the utilization of treatment in eligible patients.
References
ABS. (2012). Australian Bureau of Statistics, [website]. http://www.abs.gov.au (accessed Mar 2012).
Publisher
Adams, H. P., del Zoppo, G., Alberts, M. J., Bhatt, D. L., Brass, L., Furlan, A., Grubb, R. L., Higashida, R. T., Jauch, E. C. & Kidwell, C. (2007). “Guidelines for the Early Management of Adults with Ischemic Stroke,” Stroke, 38, 1655-1711.
Publisher – Google Scholar
Barber, P. A., Zhang, J., Demchuk, A. M., Hill, M. D. & Buchan, A. (2001). “Why are Stroke Patients Excluded from TPA Therapy? An Analysis of Patient Eligibility,” Neurology, 56, 1015-1020.
Publisher – Google Scholar
Donnan, G. A., Fisher, M., Macleod, M. & Davis, S. M. (2008). “Stroke,” The Lancet, 371, 1612-1623.
Publisher
Gilligan, A. K., Thrift, A. G., Sturm, J. W., Dewey, H. M., Macdonell, R. A. L. & Donnan, G. A. (2005). “Stroke Units, Tissue Plasminogen Activator, Aspirin and Neuroprotection: Which Stroke Intervention Could Provide the Greatest Community Benefit?,” Cerebrovascular Diseases, 20, 239-244.
Publisher – Google Scholar
Katzan, I. L., Hammer, M. D., Hixson, E. D., et al. (2004). “Utilization of Intravenous Tissue Plasminogen Activator for Acute Ischemic Stroke,” Archives of Neurology, 61, 346-350.
Publisher – Google Scholar
Kokotailo, R. A. & Hill, M. D. (2005) “Coding of Stroke and Stroke Risk Factors Using International Classification of Diseases, Revisions 9 and 10,” Stroke, 36, 1776-1781.
Publisher – Google Scholar
Krass, I., Ogle, S. J., Duguid, M. J., Shenfield, G. M. & Bajorek, B. V. (2002). “The Impact of Age on Antithrombotic Use in Elderly Patients with Non Valvular Atrial Fibrillation,” Australasian Journal on Ageing, 21, 36-41.
Publisher – Google Scholar
Leyden, J. M., Chong, W. K., Kleinig, T., et al. (2011). “A Population- Based Study of Thrombolysis for Acute Stroke in South Australia,” The Medical Journal of Australia, 194, 111-115.
Publisher – Google Scholar
NINDS. (1995). The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group.”Tissue Plasminogen Activator for Acute Ischemic Stroke,” The New England Journal of Medicine, 333, 1581-1587.
Publisher – Google Scholar
NSF 2009. National Stroke Foundation (2009). “National Stroke Audit – Acute Services Clinical Audit Report,”
Publisher
NSF 2010. National Sstroke Foundation (2010). “Clinical Guidelines for Stroke Management,”
Publisher – Google Scholar
NSF 2011. National Stroke Foundation (2011). “National Stroke Audit – Acute Services Clinical Audit Report,”
Publisher
O’Brien, W., Crimmins, D., Donaldson, W., et al. (2012). “FASTER (Face, Arm, Speech, Time, Emergency Response): Experience of Central Coast Stroke Services Implementation of a Pre-Hospital Notification System for Expedient Management of Acute Stroke,” Journal of Clinical Neuroscience, 19,241-245.
Publisher – Google Scholar
Perera, V., Bajorek, B. V., Matthews, S. & Hilmer, S. N. (2009). “The Impact of Frailty on the Utilization of Antithrombotic Therapy in Older Patients with a Trial Fibrillation,” Age and Ageing, 38, 156-162.
Publisher – Google Scholar
Quain, D. A., Parsons, M. W., Loudfoot, A. R., et al. (2008). “Improving Access to Acute Stroke Therapies: A Controlled Trial of Organised Pre-Hospital and Emergency Care,” The Medical Journal of Australia, 189, 429-433.
Publisher – Google Scholar
Rudd, A. G., Hoffman, A., Grant, R., Campbell, J. T. & Lowe, D. (2011). “Stroke Thrombolysis in England, Wales and Northern Ireland: How Much Do We Do and How Much Do We Need?,” Journal Neurology, Neurosurgery & Psychiatry, 82, 14-19.
Publisher – Google Scholar
Sandercock, P., Wardlaw, J. M., Lindley, R. I., Dennis, M., et al. (2012). “The Benefits and Harms of Intravenous Thrombolysis with Recombinant Tissue Plasminogen Activator within 6 h of Acute Ischaemic Stroke (The Third International Stroke Trial [IST-3]): A Randomised Controlled Trial,” Lancet 379, 2352-2363.
Publisher – Google Scholar
Stead, L. G., Gilmore, R. M., Bellolio, M. F., et al. (2008). “Percutaneous Clot Removal Devices in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis,” Archives of Neurology, 65, 1024-1030.
Publisher – Google Scholar
Van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J. & van Gijn, J. (1988). “Inter-Observer Agreement for the Assessment of Handicap in Stroke Patients,” Stroke, 19, 604-607.
Publisher – Google Scholar








