Introduction
Chemotherapy-related cognitive impairment (CRCI), known colloquially as chemobrain, involves a decrease in the cognitive processing capacity of an individual as a result of treatment with chemotherapy (Biglia, Bounous, Malabaila, Palmisano, Torta, D’Alonzo, Sismondi & Torta, 2012). It has been estimated to affect between 12 and 95% of all cancer patients (Downie, Mar Fan, Houede-Tchen, Yi & Tannock, 2006; Hede, 2008; Iconomou, Mega, Koutras, Iconomou & Kalofonos, 2004; Jansen, Cooper, Dodd & Miaskowski, 2011; Mehnert, Schwerath, Schirmer, Schleimer, Petersen, Schulz-Kindermann, Zander & Koch, 2007; Prokasheva, Faran, Cwikel & Geffen, 2011; Skaali, Fossa, Andersson, Cvancarova, Langberg, Lehne & Dahl, 2011). However, these estimates differ widely between studies and a general consensus has been that it affects approximately 30% of patients undergoing treatment with chemotherapeutic agents (Collins, Mackenzie, Stewart, Bielajew & Verma, 2009; Hermelink, Untch, Lux, Kreienberg, Beck, Bauerfeind & Munzel, 2007; Vardy & Dhillon, 2010).
A diverse range of cognitive domains has been thought to be possibly affected by CRCI; various forms of memory, attention and concentration, information processing speed, motor function, language, executive function and visuospatial skill (Iconomou et al., 2004; Jansen, Dodd, Miaskowski, Dowling & Cramer, 2008; Mehnert et al., 2007; Prokasheva et al., 2011; Reid-Arndt, Hsieh & Perry, 2010; Skaali et al., 2011). Although not all patients treated with chemotherapy for cancer will experience CRCI, those who do have generally reported a diminished capacity to engage in everyday tasks, with consequential reduction in quality of life (Boykoff, Moieni & Subramanian, 2009; Hede, 2008; Myers, 2009). Although such experiences can be relatively short-term, CRCI has been reported as lasting up to 10 years after treatment (Iconomou et al., 2004).
Whether significant CRCI results from chemotherapy and, if so, what mechanisms are involved is a highly debated topic within the literature. A number of mechanisms through which CRCI may arise have been proposed. For example, Jansen et al. (2008) and Myers (2009) hypothesised that CRCI occurs in response to the release of cytokines as a result of treatment. Dietrich, Han, Yang, Mayer-Proschel and Noble (2006) argued that CRCI occurs because chemotherapy is more harmful to brain cells than cancer cells and thus causes brain damage leading to cognitive impairment before the cancer cells have been eradicated. Others have also supported the notion that chemotherapy damages brain tissue, with Hampton (2008) and Meyers (2008) both claiming that CRCI occurs because of damage to the oligodendrocytes, which disrupts vital processes in the central nervous system. Other mechanisms proposed as causes of CRCI include hormonal and auto-immune responses (Meyers, 2008), damage to cerebral gray and white matter, microvasculature and DNA, and oxidative stress (Myers, 2009).
There are a number of variables that have been reported to influence the occurrence of CRCI in patients with cancer. Specifically, it is thought that CRCI is less likely to occur and, if it does occur is less severe, in those patients who have higher premorbid IQ or who have received a higher level of education (Jansen et al., 2011). Alternatively, a propensity towards greater anxiety, depression and fatigue, and chemotherapy-induced anaemia and menopause are thought to increase the likelihood and exacerbate the symptoms of CRCI (Jansen et al., 2011).
However, when conducting research into possible chemotherapy-related cognitive impairment, it is important to bear in mind that any impairment observed may not be due to the chemotherapy at all. CRCI research also overlaps with an area of research that investigates postoperative cognitive dysfunction (POCD). This is important because most cancer patients being treated with chemotherapy have also undergone surgical treatment for their cancer and POCD, much like CRCI, may involve a reduction in the cognitive abilities of a patient as a consequence of surgical intervention.
The cause of POCD has been debated within the literature; it is thought to occur either due to the effects of general anaesthetics on the brain (Avidan & Evers, 2011, Chen, Zhao, White, Li, Tang, Wender, Sloninsky, Naruse, Kariger, Webb & Norel, 2001), or as a consequence of the actions of the inflammatory system on brain functioning (Avidan & Evers, 2011, Cibelli, Fidalgo, Terrando, Ma, Monaco, Feldmann, Takata, Lever, Nanchahal, Fanselow & Maze, 2010). There is also debate surrounding the duration of POCD, with estimates ranging from a few days up to three months post-surgery (Avidan & Evers, 2011, Moller, Cluitmans, Rasmussen, Houx, Rasmussen, Canet, Rabbitt, Jolles, Larsen, Hanning, Langeron, Johnson, Lauven, Kristensen, Biedler, van Beem, Fraidakis, Silverstein, Beneken & Gravenstein, 1998), durations consistent with some reports of CRCI although much shorter than the longer estimates sometimes claimed. It has, however, been established that POCD is both more prevalent and more severe among the older population and that these individuals are more likely to suffer its effects for longer and have a reduced quality of life as a result (Avidan & Evers, 2011, Chen et al., 2001, Moller et al., 1998). POCD is relevant to this study because it is possible that CRCI could be confused with POCD in patient groups treated using surgical methods in combination with chemotherapy. Therefore, a surgery-only treatment for cancer group was included in the present study, in order to evaluate this possibility.
It has been noted previously that objective neuropsychological tests may not be sufficiently sensitive to reflect the problems encountered in the everyday lives of patients and that, as a result, there is a discrepancy between the findings of objective testing and subjective impressions (self-report, others’ opinions) in this area (Downie et al., 2006). Consequently, Hutchinson, Hosking, Kichenadasse, Mattiske and Wilson (2012) have called for the inclusion of a test that assesses everyday problem solving when evaluating the utility of neuropsychological assessment for measuring CRCI. In order to build upon the findings of existing studies, the present study will address this concern by including the Everyday Problems Test (EPT) – a measure of problem solving abilities in situations regularly encountered in everyday lives.
Colorectal cancer, also commonly known as bowel cancer, is a highly prevalent form of cancer in Australia; second only to breast cancer in women and prostate cancer in men (Bowel Cancer Australia, 2010; Cancer Council Australia, 2009). Specifically, by the age of 85 years, one in 10 Australian men and one in 15 Australian women will have been diagnosed with colorectal cancer. Due to improved and widely promoted screening techniques, the rates of survival associated with this form of cancer are ever increasing but survival rates decrease with increasing severity. To be precise, when diagnosed with early stage colorectal cancer, which requires only surgical treatment, one is currently expected to have an 87-90% chance of survival. Those with stage 3 of the disease, which usually involves both surgery and treatment with chemotherapy, are expected to have approximately a 57% chance of survival. Widespread stage 4 colorectal cancer, which can only be treated using chemotherapy, is associated with around a 10% chance of survival (Cancer Council Australia, 2009). Therefore, as a result of the high incidence of colorectal cancer in Australia in conjunction with the improving rates of survival if detected early, it is critically important to investigate the effects of treatment with chemotherapy on cognition in patients who have been diagnosed with this disease.
This study has aimed to test whether treatment with chemotherapy leads to cognitive impairment in patients with colorectal cancer. This is important because, despite its high prevalence, the effect of treatment on the cognitive functioning of patients with colorectal cancer is yet to be evaluated despite the extensive literature predominantly investigating this phenomenon in breast cancer. Specifically, we assessed the effect of treatment with chemotherapy on cognition in patients with colorectal cancer and compared these effects with those of other treatments for this type of cancer including surgical treatment alone and treatment with anti-angiogenic drugs. The hypotheses tested were: (1a) that treatment with chemotherapy or chemotherapy and the anti-vascular drug leads to impairment across a number of cognitive tests for attention, memory and processing speed by comparison to matched controls, and (1b) that cognitive impairment will also be evident in the surgery-only control group by comparison to matched controls as a result of POCD.
In addition, consistent with the literature, it was hypothesised that (2) those with greater levels of depression, anxiety and fatigue will exhibit worse cognitive function; (3) those with a higher level of education and/or higher premorbid ability will display higher cognitive functioning; and, (4) participants with higher scores on the Everyday Problems Test will have better cognitive functioning. It should be noted that impairment was measured as the average performance of each of the three treatment groups relative to that of a healthy, age- and education-matched control group.
Method
Patients with a diagnosis of colorectal cancer, treated with either surgery alone, chemotherapy with surgery and without surgery, or chemotherapy with the anti-angiogenic drug Avastin (bevacizumab) with and without surgery between October 2009 and April 2012 were recruited through the oncology departments at Flinders Medical Centre and the Royal Adelaide Hospital. To be included in the present study, patients were required to be aged over 50 years, have received a minimum of three months of chemotherapy, with or without Avastin, and in the case where treatment had already been completed, be no more than one month post-treatment. Surgery patients were also required to be no more than one month post treatment. Patients were excluded if they had been treated with chemotherapy for any other instances of cancer, had a current diagnosis of anxiety or depression, or had a history of head injury, stroke, drug or alcohol abuse, or of a neurological or psychiatric condition. Healthy control participants were recruited through word-of-mouth at the two hospitals or were contacted from among people who had participated in previous unrelated research, run through the University of Adelaide. Healthy control participants, without the diagnosis/ treatment for colorectal cancer, were also required to conform to the same inclusion and exclusion criteria as the patient groups. Ethics approval was obtained through the University of Adelaide, Flinders Medical Centre and Royal Adelaide Hospital Human Research Ethics Committees.
Eligible patients were first introduced to the study by their oncologist and provided with an information sheet. If interested in participating, their details were passed to the first author. They were contacted by phone to schedule a time to participate. The testing session took place either in the hospital at which the participant was receiving treatment, at their home, or at the University of Adelaide. At the testing session, participants were again provided with the information sheet, a consent form and instructed to read these and if willing to participate, provide consent. Participants were informed that they were free to withdraw from the study at any time without consequence. No one withdrew.
Testing began with the Everyday Problems Test (EPT), which was a 21-item multiple choice measure that assessed the extent to which a person can solve problems most likely encountered on a regular basis in their everyday lives, such as following recipes and filling out forms. Participants had a maximum of 20 minutes to complete this assessment. The EPT is both a reliable and valid measure with high test-retest reliability (0.83 – 0.91) and construct validity (0.42 – 0.72) (Willis & Marsiske, 1993). Participants subsequently completed a number of scales assessing depression, anxiety and fatigue; the Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI) and Fatigue Assessment Scale. In addition, participants completed the Functional Assessment of Cancer Therapy-Cognitive (FACT-Cog) and Functional Assessment of Cancer Therapy-Colorectal (FACT-C) subscales assessing self-reported cognitive functioning and quality of life subsequent to a diagnosis of cancer. The results of these self-report measures are not discussed here.
Following these questionnaires neuropsychological assessment involved the Trail Making Test (TMT), followed by the Controlled Oral Word Association Test (COWAT), Rey Auditory Verbal Learning Test (RAVLT), Digit Span test, Rey Complex Figure Test (RCFT), the Logical Memory test from the Wechsler Memory Scale III and the Inspection Time task (IT). Finally, the Wechsler Test of Adult Reading (WTAR) assessed premorbid intelligence, i.e. the predicted level of intelligence of the participants before they became ill based on their ability to pronounce words, some unknown to them, which is not affected by illness or treatment; followed by the Stroop test and Digit Symbol from the Wechsler Adult Intelligence Scale III. Table 1 describes the neuropsychological abilities evaluated by these tests as determined by their respective publishers; indicated in the manuals.
Table 1. Cognitive Abilities Assessed by the Nine Neuropsychological Tests
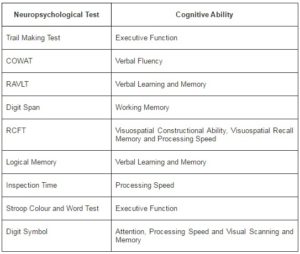
COWAT = Controlled Oral Word Association Test, RAVLT = Rey Auditory Verbal Learning Test, RCFT =
Rey Complex Figure Test.
All neuropsychological tests were administered and scored according to the instructions outlined in their respective manuals. Each measure has been shown to be reliable and valid; the COWAT has a test-retest reliability of 0.70, while the RAVLT has an internal consistency of 0.70 for list A (Snow, Tierney, Zorzitto, Fisher & Reid, 1988). Digit span and logical memory have demonstrated test-retest reliabilities of 0.84-0.93 and 0.74-0.91, respectively, depending on age group (Tulsky, Zhu & Ledbetter, 1997). The Stroop test was shown to have a test-retest reliability of 0.73 for the colour-word component (Jensen, 1965), while that for Digit Symbol was 0.84-0.87, depending on the age group of participants (Tulsky, Zhu & Ledbetter, 1997). The RCFT had a test-retest reliability of 0.76 for the copy component and 0.89 for the recall component (Meyers & Meyers, 1995), while test-retest reliability for the Inspection Time task is usually 0.80 and higher (Grudnik & Kranzler, 2001). Each of these tests correlated well with other tests measuring the same construct. No reliability or validity data were available for the Trail Making Test. The results of this study were analysed using the Statistical Package for the Social Sciences (SPSS) version 18.
Results
Comparison between Treatment Groups
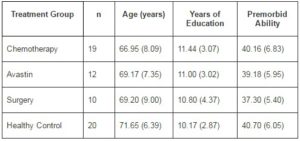
Sixty-one patients were recruited to participate in this study (30 male). Of these, 19 were treated with chemotherapy, an additional 12 with Avastin (bevacizumab), 10 received only surgical intervention and 20 were age- and education-matched healthy controls. The decision was made to include 20 healthy controls based on the fact that the largest cancer treatment group consisted of only 19 patients and having relatively equal numbers in the chemotherapy and control groups was a goal of this research. The chemotherapy group comprised 17 patients who had been treated with surgery and chemotherapy and two participants who were treated using only chemotherapy. Of the Avastin group, seven were treated using surgery, chemotherapy and Avastin, while the other five were treated with chemotherapy and Avastin. As described above, participants in both the chemotherapy and Avastin treatment groups received chemotherapy. More specifically, nine participants were treated with oxaliplatin, calcium folinate and 5-flourouracil, eight using capecitabine (xeloda), six with calcium folinate and 5-flourouracil, and one with capecitabine, calcium folinate, oxaliplatin and 5-flourouracil, capecitabine, 5-flourouracil and calcium folinate, and capecitabine and oxaliplatin, respectively. Group-specific descriptive statistics for age, level of education and premorbid ability are presented in Table 2. Univariate analyses of variance revealed no significant differences between the four groups in age, years of education and premorbid ability.
Table 2. Means and Standard Deviations for Age, Level of Education and Premorbid Ability
To investigate whether there was a difference between the four groups in terms of their cognitive performance a MANOVA was conducted using the subscale scores from each neuropsychological test. This analysis revealed a significant difference between the four groups in cognitive functioning (F (45, 81) = 1.12, p < 0.01). Post hoc testing showed that this difference was between the surgery and healthy control groups in the second recall component of the Logical Memory test. As can be seen in Table 3, the surgery group performed more poorly on this task than the healthy control group. The groups did not significantly differ in their performance on any of the other subtests. Descriptive statistics for these MANOVAs are displayed in Table 3. These results do not provide support for the first hypothesis that treatment (surgery or chemotherapy) for colorectal cancer would lead to cognitive impairment. Due to the large number of comparisons across the four groups for the neuropsychological tests, it is likely that the one significant result occurred because of a Type II error rather than an actual effect.
In order to further evaluate whether treatment with chemotherapy leads to cognitive impairment, the chemotherapy and Avastin groups were combined to form one larger chemotherapy treatment group (n = 31). A MANOVA was conducted to establish whether or not there were any differences between this group, the surgery group (n = 10) and healthy controls (n = 20) in performance on the neuropsychological tests. This revealed no difference for the total neuropsychological test scores (F (20, 58) = 1.12, p = 0.36) or for the subtest scores (F (30, 56) = 1.26, p = 0.22). These results also fail to provide partial support for the first hypothesis. See Table 3 for descriptives.
Table 3. Descriptive Statistics for Performance on Neuropsychological Tests of the Chemotherapy, Avastin, Surgery, Healthy Control and Chemotherapy and Avastin Combined Groups
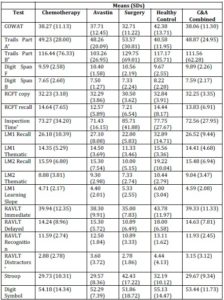
*For the Trail Making Test, Inspection Time and RAVLT Distractors, a lower score indicates better performance.
Emotional Functioning
Correlation analyses evaluated relationships between depression, anxiety, fatigue and cognitive functioning. Depression and fatigue were not significantly related to performance on any of the neuropsychological tests, and anxiety was related only to performance on the Trail Making Test (r = 0.25, p = 0.05); higher self reported fatigue was associated with poorer Trail Making performance. Taken together, these results fail to support the hypothesis (2) that depression, anxiety and fatigue would be related to poorer cognitive functioning. Additionally, further correlations were conducted to examine the relationship between depression, anxiety and fatigue and performance on the neuropsychological tests for each of the five groups. Table 4 displays these correlations; most of which are in the opposite direction to what was predicted by the second hypothesis. Fatigue was related to cognitive performance in the chemotherapy group however, this was only on a single subscale of two respective tests; anxiety was correlated with neuropsychological test performance for the Trail Making and Inspection Time tasks in the Avastin treatment group, while part A of the Trail Making Test was related to anxiety in the chemotherapy and Avastin combined group (Table 4).
Correlations were undertaken to assess whether years of education or premorbid intelligence were associated with cognitive function; significant positive correlations were found between both years of education and premorbid ability and a number of neuropsychological tests, as set out in Table 5. Hypothesis 3, that more education and premorbid ability will be related to better cognitive functioning, is therefore supported.
The Everyday Problems Test (EPT) was correlated with the different neuropsychological test scores to assess whether functioning in everyday life situations was related to neuropsychological test performance. Statistically significant correlations were revealed between the EPT and each of the neuropsychological tests, with the exception of the Stroop Colour and Word Test (see Table 6). This confirms hypothesis 4, that everyday problem solving ability and cognitive functioning are related.
Table 4. Relationships between Depression, Anxiety and Fatigue and Neuropsychological Test Performance across the Five Groups

Table only contains significant results. *lower scores indicate superior performance. C&A = Chemotherapy +
Avastin combined to form one drug treatment group.
Table 5. Significant Correlations between Years of Education and Premorbid Ability and the Neuropsychological Tests
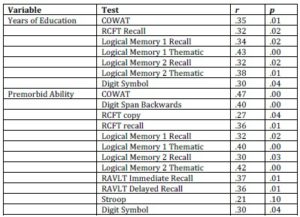
Table 6. Significant Correlations between the EPT and Neuropsychological Tests
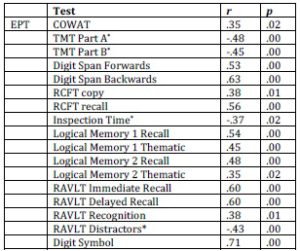
*In contrast to the other tests, lower scores on the Trail Making Test, Inspection
Time task and RAVLT Distractors indicate better performance.
Discussion
This study examined the effect of chemotherapy on cognition in patients with colorectal cancer. Hypothesis 1 was not supported because patients being treated with chemotherapy or Avastin or with only surgical intervention did not perform statistically significantly worse than the healthy controls. This study therefore revealed no evidence of chemotherapy-related cognitive impairment (CRCI), post-operative cognitive dysfunction (POCD), or any other treatment- or cancer-related cognitive impairment in patients with colorectal cancer. These findings are not consistent with the literature, which states that between 12 and 95% of patients treated with chemotherapy for other types of cancers do experience symptoms of CRCI (Downie, Mar Fan, Houede-Tchen, Yi & Tannock, 2006; Hede, 2008; Iconomou, Mega, Koutras, Iconomou & Kalofonos, 2004; Jansen, Cooper, Dodd & Miaskowski, 2011; Mehnert, Schwerath, Schirmer, Schleimer, Petersen, Schulz-Kindermann, Zander & Koch, 2007; Prokasheva, Faran, Cwikel & Geffen, 2011; Skaali, Fossa, Andersson, Cvancarova, Langberg, Lehne & Dahl, 2011). Limited literature on the issue of CRCI in patients with colorectal cancer illustrates that lack of CRCI (Andreis, Ferri, Mazzocchi, Meriggi, Rizzi, Rota, et al., 2012). Thus it is possible that CRCI simply does not exist in this patient group. The possible mechanism behind the lack of CRCI needs further investigations despite animal studies with similar chemotherapy drugs demonstrating cognitive impairment.
The second hypothesis, that participants with greater depression, anxiety and fatigue would exhibit lower levels of cognitive functioning was not supported. A significant correlation was revealed only between anxiety and performance on the Trail Making Test; however anxiety was unrelated to the other measures of cognitive functioning while depression and fatigue were not related to performance on any of the neuropsychological tests. This is also inconsistent with literature that has found that cognitive functioning declines in those with depression, anxiety and fatigue (Jansen et al., 2011).
Hypothesis three, that participants with more years of education and greater premorbid ability will exhibit better cognitive functioning, was confirmed. Statistically significant relationships were established between years of education and premorbid ability and a number of the cognitive tests. This is consistent with the existing literature. Jansen et al. (2011) also found that those with higher levels of education and intelligence tended to perform better on neuropsychological tests and retain a higher level of cognitive functioning compared to those with lower levels of these.
The fourth hypothesis, that those scoring higher on the Everyday Problems Test (EPT) will also demonstrate better cognitive functioning was also confirmed. Statistically significant correlations were established between the EPT and all of the neuropsychological tests, with the exception of the Stroop Colour and Word Test. This is an important finding in informing the literature. Past studies have called for research to be conducted into the relationship between everyday problem solving abilities and cognitive functioning in cancer patients (Hutchinson et al., 2012). This study has shown that neuropsychological tests are good predictors of the problems participants come across in their everyday lives and there are moderate to strong relationships between these two variables. Therefore, based on the findings of the present study, the recommendation can be made that it is acceptable for traditional objective neuropsychological tests to be used to assess the effects of CRCI in cancer patients because they are positively related to everyday problem solving ability (as evidenced by the EPT).
Limitations of Study
The present study had a number of limitations. A larger sample size and having a similar number of participants in each of the four groups, who were homogeneous in the treatment they received, would have provided the study with greater statistical power and, in turn, made the results more reliable and generalisable to the colorectal-cancer patient population. Therefore, due to its small sample size, the results of the present study must be interpreted with caution. Future studies should aim to recruit a much larger number of participants in order to produce more reliable data and would therefore provide the researcher with the opportunity to delete participants with missing cases if that situation arose.
To conclude, this study investigated the effect of treatment with chemotherapy, Avastin and surgery on cognition in patients with colorectal cancer, with none of the three treatment groups exhibiting cognitive impairment relative to the healthy controls. The effects of level of education, premorbid ability and everyday problem solving on cognitive functioning in these patients were also investigated, with statistically significant correlations being found between each of these and many of the cognitive tests. Depression, anxiety and fatigue were established as being unrelated to cognitive functioning in this patient group. Future studies should investigate the effects of chemotherapy on cognition in a larger cohort of colorectal cancer patients, as well as examining further the relationship between everyday problem solving, using the EPT and/or alternative instruments, in cancer patients generally.
(adsbygoogle = window.adsbygoogle || []).push({});
Reference
Andreis, F., Ferri, M., Mazzocchi, M., Meriggi, M., Rizzi, A., Rota, L., Di Biasi, B., Abeni, C., Codignola , C. & Rozzini, R., et al. (2012). Lack of a Chemobrain Effect for Adjuvant FOLFOX Chemotherapy in Colon Cancer Patients. A Pilot Study. Support Care Cancer, Published Online.
Publisher – Google Scholar
Avidan, M. S. & Evers, A. S. (2011). “Review of Clinical Evidence for Persistent Cognitive Decline or Incident Dementia Attributable to Surgery or General Anaesthesia,” Journal of Alzheimer’s Disease, 24(2), 201-216.
Publisher
Biglia, N., Bounous, V. E., Malabaila, A., Palmisano, D., Torta, D. M., D’Alonzo, M., Sismondi, P. & Torta, R. (2012). “Objective and Self-Reported Cognitive Dysfunction in Breast Cancer Women Treated with Chemotherapy: A Prospective Study,” European Journal of Cancer Care, 21, 485-492.
Publisher – Google Scholar
Bowel Cancer Australia (2010), Retrieved July 4, 2012 from http://www.bowelcanceraustralia.org/bca/index.php?option=com_content&view=article&id=163&Itemid=289#facts.
Publisher
Boykoff, N., Moieni, M. & Subramanian, S. K. (2009). “Confronting Chemobrain: An in-Depth Look at Survivors’ Reports of Impact on Work, Social Networks, and Health Care Response,” Journal of Cancer Survivorship, 3, 223 – 232.
Publisher – Google Scholar
Cancer Council Australia (2009) Colorectal Cancer, Retrieved April 21, 2010 from http://www.cancer.org.au//aboutcancer/cancertypes/colorectalcancer.htm.
Chen, X., Zhao, M., White, P. F., Li, S., Tang, J., Wender, R. H., Sloninsky, A., Naruse, R., Kariger, R., Webb, T. & Norel, E. (2001). “The Recovery of Cognitive Function after General Anaesthesia in Elderly Patients: A Comparison of Desflurane and Sevoflurane,” Anaesthesia and Analgesia, 93, 1489-1494.
Publisher – Google Scholar – British Library Direct
Collins, B., Mackenzie, J., Stewart, A., Bielajew, C. & Verma, S. (2009). “Cognitive Effects of Chemotherapy in Post-Menopausal Breast Cancer Patients 1 Year after Treatment,” Psycho-Oncology, 18, 134 – 143.
Publisher – Google Scholar
Dietrich, J., Han, R., Yang, Y., Mayer-Proschel, M. & Noble, M. (2006). “CNS Progenitor Cells and Oligodendrocytes Are Targets of Chemotherapeutic Agents in Vitro and in Vivo,” Journal of Biology, 5, 22.1 – 22.23.
Publisher
Downie, F. P., Mar Fan, H. G., Houede-Tchen, N., Yi, Q. & Tannock, I. F. (2006). “Cognitive Function, Fatigue and Menopausal Symptoms in Breast Cancer Patients Receiving Adjuvant Chemotherapy: Evaluation with Patient Interview after Formal Assessment,” Psycho-Oncology, 15, 921-930.
Publisher – Google Scholar – British Library Direct
Grudnik, J. L. & Kranzler, J. H. (2001). “Meta-Analysis of the Relationship between Intelligence and Inspection Time,”Intelligence, 29, 523 – 536.
Publisher – Google Scholar – British Library Direct
Hampton, T. (2008). “Studies Reveal Underlying Mechanism for Chemotherapy’s Adverse Effects on Brain,” Journal of the American Medical Association, 299, 2494.
Publisher – Google Scholar
Hede, K. (2008). “Chemobrain Is Real But May Need New Name,” Journal of the National Cancer Institute, 100, 162 – 169.
Publisher – Google Scholar – British Library Direct
Hermelink, K., Untch, M., Lux, M. P., Kreienberg, R., Beck, T., Bauerfeind, I. & Munzel, K. (2007). “Cognitive Function during Neoadjuvant Chemotherapy for Breast Cancer,” Cancer, 109, 1905 – 1913.
Publisher – Google Scholar – British Library Direct
Hutchinson, A. D., Hosking, J. R., Kichenadasse, G., Mattiske, J. K. & Wilson, C. (2012). “Objective and Subjective Cognitive Impairment Following Chemotherapy for Cancer: A Systematic Review,” Cancer Treatment Reviews, Available Online.
Publisher – Google Scholar
Iconomou, G., Mega, V., Koutras, A., Iconomou, A. V. & Kalofonos, H. P. (2004). “Prospective Assessment of Emotional Distress, Cognitive Function, and Quality of Life in Patients with Cancer Treated with Chemotherapy,” Cancer, 101(2), 404-411.
Publisher – Google Scholar – British Library Direct
Jansen, C. E., Cooper, B. A., Dodd, M. J. & Miaskowski, C. A. (2011). “A Prospective Longitudinal Study of Chemotherapy-Induced Cognitive Changes in Breast Cancer Patients,” Support Care Cancer, 19, 1647-1656.
Publisher – Google Scholar
Jansen, C. E., Dodd, M. J., Miaskowski, C. A., Dowling, G. A. & Kramer, J. (2008). “Preliminary Results of a Longitudinal Study of Changes in Cognitive Function of Breast Cancer Patients Undergoing Chemotherapy with Doxorubicin and Cyclophosphamide,” Psycho-Oncology, 17, 1189-1195.
Publisher – Google Scholar
Jensen, A. R. (1965). “Scoring the Stroop Test” Acta Psychologia, 24, 398 – 408.
Publisher – Google Scholar
Lezak, M. D., Howieson, D. B. & Loring, D. W. (2004). Neuropsychological Assessment (4 Ed.), New York: Oxford University Press.
Publisher – Google Scholar
Mehnert, A., Scherwath, A., Schirmer, L., Schleimer, B., Petersen, C., Schulz-Kindermann, F., Zander, A. R. & Koch, U. (2007). “The Association between Neuropsychological Impairment, Self-Perceived Cognitive Deficits, Fatigue and Health-Related Quality of Life in Breast Cancer Survivors Following Standard Adjuvant Versus High Dose Chemotherapy,” Patient Education and Counselling, 66, 108-118.
Publisher – Google Scholar
Meyers, C. A. (2008). “How Chemotherapy Damages the Central Nervous System,” Journal of Biology, 7, 11.1 – 11.3.
Publisher – Google Scholar
Meyers, J. E. & Meyers, K. R. (1995). ‘Rey Complex Figure Test and Recognition Trial: Professional Manual,’ Odessa, FL: Psychological Assessment Resources.
Google Scholar
Moller, J. T., Cluitmans, P., Rasmussen, L. S., Houx, P., Rasmussen, H., Canet, J., Rabbitt, P., Jolles, J., Larsen, K., Hanning, C. D., Langeron, O., Johnson, T., Lauven, P. M., Kristensen, P. A., Biedler, A., Van Beem, H., Fraidakis, O., Silverstein, J. H., Beneken, J. E. & Gravestein, J. S. (1998). “Long-Term Post-Operative Cognitive Dysfunction in the Elderly ISPOCD1 Study,” Lancet, 351, 857-861.
Publisher – Google Scholar – British Library Direct
Myers, J. S. (2009). “Chemotherapy-Related Cognitive Impairment: Neuroimaging, Neuropsychological Testing, and the Neuropsychologist,” Clinical Journal of Oncology Nursing, 13, 413 – 421.
Publisher – Google Scholar
Prokasheva, S., Faran, Y., Cwikel, J. & Geffen, D. B. (2011). “Analysis of Memory Deficits Following Chemotherapy in Breast Cancer Survivors: Evidence from the Doors and People Test,” Journal of Psychosocial Oncology, 29, 499-514.
Publisher – Google Scholar
Reid-Arndt, S. A., Hsieh, C. & Perry, M. C. (2010). “Neuropsychological Functioning and Quality of Life During the First Year after Completing Chemotherapy for Breast Cancer,” Psycho-Oncology, 19, 535-544.
Publisher – Google Scholar
Skaali, T., Fossa, S. D., Andersson, S., Cvancarova, M., Langberg, C. W., Lehne, G. & Dahl, A. A. (2011). “Self-Reported Cognitive Problems in Testicular Cancer Patients: Relation to Neuropsychological Performance, Fatigue, and Psychological Distress,” Journal of Psychosomatic Research, 70, 403-410.
Publisher – Google Scholar
Snow, W. G., Tierney, M. C., Zorzitto, M. L., Fisher, R. H. & Reid, D. W. (1988). ‘One-Year Test-Retest Reliability of Selected Tests in Older Adults,’ Paper Presented at the Meeting of the International Neuropsychological Society, New Orleans.
Tulsky, D., Zhu, J. & Ledbetter, M. F. (1997). ‘WAIS-III WMS-III Technical Manual,’ San Antonio: The Psychological Corporation.
Google Scholar
Vardy, J. & Dhillon, H. (2010). “The Fog Hasn’t Lifted on “Chemobrain” Yet: Ongoing Uncertainty Regarding the Effects of Chemotherapy and Breast Cancer on Cognition,” Breast Cancer Research and Treatment, 121.
Publisher – Google Scholar
Willis, S. L. & Marsiske, M. (1993). ‘Manual for the Everyday Problems Test, University Park: Department of Human Development and Family Studies,’ Pennsylvania State University.








