Introduction
Spinal cord injury (SCI) consists in an insult to the spinal cord, resulting in temporary or permanent impairment of sensorimotor and autonomic functions. It is an invalidating pathology, unfortunately without effective treatments, even though researchers are currently evaluating a number of therapies to promote axonal regrowth and to restore connectivity (Thuret et al., 2006).
Among these, environmental enrichment (EE) housing seems a promising approach: animals are stimulated in their cages by the presence of plastic tubing, running wheels and toys. EE potentiates social interactions (animals are housed in group) and sensorimotor activity (Fischer and Peduzzi, 2007). EEc an induce improvements in the diseased brain, as shown in several animal models of Alzheimer’s, Parkinson’s and Huntington’s diseases (Nithianantharajah and Hannan, 2006). Additionally, EE promotes neurogenesis and cell integration into existing circuits, induces dendritic branching and length, increases the number of dendritic spines and synapses (Nithianantharajah and Hannan, 2006).
Similarly, exercise training can provide important benefits after SCI: indeed, both approaches can exert positive effects on functional recovery after experimental SCI (Berrocal et al., 2007; Engesser-Cesar et al., 2007), in particular due to increased levels of neurotrophins, GDNF (glial cell-derived neurotrophic factor), NGF (nerve growth factor) and BDNF (brain-derived neurotrophic factor; Lewis et al., 2004; Nithianantharajah and Hannan, 2006; Olson et al., 2006; Wolf et al., 2006; Kobilo et al., 2011).
Another relevant source of growth factors is represented by stem cells, which can release BDNF, NGF, GDNF, VEGF (vascular endothelial growth factor), NT3 (neurotrophin 3), IGF (insuline-like growth factor), essential for neurons and regenerating fibers after an insult (Rossi and Keirstead, 2009; Garbossa et al., 2012).
Here we have investigated the concurrent use of stem cells and enrichment/running in a murine model of spinal cord compression, as a potential therapeutic approach, in order to enhance the respective benefits. The few studies relative to this combined approach, respectively applied into a rodent model of Huntington’s disease (Döbrössy and Dunnett, 2006) and focal ischemia (Hicks et al., 2007), showed encouraging results concerning improved functional recovery and plasticity. The rationale for cotransplantation consists in aiming to a synergistic effect of two different kinds of cells to promote neural regeneration and recovery of locomotor functions.
We decided to use neural precursors (NPs), including neuronal- and glial-restricted precursors, obtained from murine neural tube (Mujtaba et al., 1999), and mesenchymal stem cells (MSCs) collected from adult bone marrow and expandedin vitro until transplantation (Tropel et al., 2004). We have already employed such cells (separately, but at higher concentration), following hemisection or compression injury and at different therapeutic time windows (acute or delayed): in any conditions, NPs and MSCs were able to exert beneficial effects concerning the sprouting of raphespinal axons, the reduction of glial cyst, and the functional motor recovery (Boido et al., 2009; Boido et al., 2011).
At our knowledge, this is the first report on the synergistic effects of cotransplantation of NPs and MSCs, and EE/exercise condition, in a murine model of spinal cord compression.
Materials and Methods
Experimental Animals
We produced SCI on adult C57BL/6J male mice (weight range 22-32 g) purchased from Janvier (Le Genest-Saint-Isle, France). Animals had free access to food and water. All experimental procedures on live animals were performed according to the European Communities Council Directive of 24 November 1986 (86/609/EEC), authorization number 17/2010-B of 30 June 2010 by Italian Department of Health, University of Torino’s institutional guidelines on animal welfare (DL 116/92). All efforts were made to minimize the number of animals used and their suffering: the total number of animals employed for surgery was 44.
Other 20 C57BL/6J male mice were specifically sacrificed in order to collect MSCs for transplantation.
On the other hand, NPs for transplantation were obtained from BCF1 mice that express Enhanced Green Fluorescent Protein (EGFP) under the beta-actin promoter, kindly provided by Dr. M. Okabe (Osaka University, Suita, Japan; Okabe et al., 1997) and bred in our animal facility: 4 pregnant mice at E12 were killed by cervical dislocation and NPs for transplantation were obtained from the embryonic neural tube (20 embryos).
Isolation and Culture of MSCs
We produced SCI on adult C57BL/6J male mice (weight range 22-32 g) purchased from Janvier (Le Genest-Saint-Isle, France). Animals had free access to food and water. All experimental procedures on live animals were performed according to the European Communities Council Directive of 24 November 1986 (86/609/EEC), authorization number 17/2010-B of 30 June 2010 by Italian Department of Health, University of Torino’s institutional guidelines on animal welfare (DL 116/92). All efforts were made to minimize the number of animals used and their suffering: the total number of animals employed for surgery was 44.
Other 20 C57BL/6J male mice were specifically sacrificed in order to collect MSCs for transplantation.
On the other hand, NPs for transplantation were obtained from BCF1 mice that express Enhanced Green Fluorescent Protein (EGFP) under the beta-actin promoter, kindly provided by Dr. M. Okabe (Osaka University, Suita, Japan; Okabe et al., 1997) and bred in our animal facility: 4 pregnant mice at E12 were killed by cervical dislocation and NPs for transplantation were obtained from the embryonic neural tube (20 embryos).
Isolation of NPs
EGFP-positive pregnant mice at E12 were killed by cervical dislocation: EGFP-positive embryos(n = 20) were isolated and kept in chilled saline. The caudal portion of neural tube was isolated and mechanically triturated with a Pasteur pipette (Mujtaba et al., 1999).Once dissociated, the cells were counted on a Burker chamber and re-suspended in saline solution at a final concentration of 33.3×103 cells/μl.
Surgery and Cell Injection Procedures
Mice were assigned to the following groups: (i) spinal cord injury (SCI) + cotransplantation (stem cell cocktail with 2/3 NPs and 1/3 MSCs, re-suspended in 2 μl of saline solution) + EE/exercise (ICoE Group — Injury Cotransplantation EE/Exercise), n = 7; (ii) SCI + Cotransplantation (ICo Group), n = 5; (iii) SCI + saline + EE/exercise (IE Group — Injury EE/Exercise), n = 10; (iv) SCI + saline solution (IS Group — Injury Saline), n = 8. Compared to the total number of injured animals (n = 44), the final number of included mice in the present study was reduced (n = 30): we excluded 14 animals in which either we did not find survived grafted cells, or the lesion size was incompatible (too small/too big) with the used injury model (see below).
Spinal cord compression was induced according Farooque (2000). Briefly, adult C57BL/6J mice were deeply anaesthetized with 3% isoflurane vaporized in O2/N2O 50:50. A laminectomy was performed at the level of the T13 vertebra, corresponding to the L2 neuromer. The compression was applied onto the spinal cord for 5 min, gently laying around plate (diameter 1.5 mm), which was longitudinally oriented over the dorsal aspect of spinal cord, weighing 10 g, to produce moderate injury. The plate was then removed: the animals were sutured and the wound was disinfected.
Two weeks after SCI, the animals ICoE and ICowere re-anaesthetized and the stem cell cocktail (2 μl containing 105cells in saline in the following proportions: 2/3 NPs and 1/3 MSCs) was slowly (over about 60 seconds) injected into the spinal cord at the same level as above into the SCI lesion cavity, via a glass micropipette (outer tip diameter 50 μm). IE and IS mice received the vehicle alone (Fig. 1).
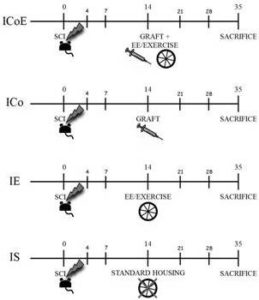 Figure 1 — Study Design
Figure 1 — Study Design
SCI was performed at day 0 in every group. Fourteen days after SCI, ICoE mice received NP/MSC graft and were housed in enriched cages (with running wheel, hiding house and tubing); ICo mice were simply grafted as ICoE; IE mice were not transplanted, but were housed in enriched cages; finally IS mice were simply housed in standard cages, until sacrifice on 35th day after SCI. Every group underwent a battery of behavioral tests before surgery and at 4-7-14-21-28-35 days post injury.
Enriched Environment Housing
Immediately after the graft, the animals were housed in either a standard cage or an enriched environment, depending on their group of assignment (Fig. 1). In standard cages, animals were group-housed (up to 5 per cage) in absence of enrichment objects. In enriched cages, the animals were group-housed and were provided with various enrichment objects (e.g. running wheel, hiding house and tubing) that were changed and rearranged once a week. Additionally, in order to force the animals to run, every day the ICoE and IE mice were forcedly put on the wheel for getting some exercise for 5 minutes.
Behavioral Testing
Injured mice (ICoE n = 7; ICo n = 5;IE n = 10; IS n = 8) underwent a battery of behavioral tests before surgery to establish a baseline for comparison withpost-surgicalvalues, studied at 4-7-14-21-28-35 days post injury, and also to evaluate the recovery promoted by stem cells and EE/exercise (from 14th day post injury): Basso Mouse Scale (BMS),foot-fault test, hindlimb flexion test and sensory test. All behavioral tests were performed by two researchers who were ‘‘blinded’’ as to the group to which the mouse belonged.
BMS – Hindlimb motor function was assessed in an open field, using the 9-point BMS locomotor rating scale: the gait of the mouse was observed for 4 min, categorizing hindlimb joint movements, trunk position and stability, stepping coordination, paw placement, the clearance and tail position, as described in Basso et al. (2006).
Foot-fault test – Mice were placed on elevated grids. Mice put their paws on the wire while moving along the grid. With each weight-bearing step, the paw may fall or slip between the wire. This was recorded as a foot-fault. The observers counted the total number of steps (N = 30) and the total number of foot-faults (Pitsikas et al., 2001; Zhang et al., 2002).
Hindlimb flexiontest- The animal’s head was covered with a hand and the hindlimbs were gently pulled toward the tail and turned over the sole. If retractive power was the same for both hindlimbs, the score would be 0; if the hindlimb retractive power was weaker than normal, the score was 1; if the lesioned hindlimbs were extended abnormally, but were retractable when the sole was touched with a finger, the score was 2; if the hindlimbs were extended abnormally and were not retractable when the sole was touched with a finger, the score was 3(Takamatsu et al., 2002).
Sensory test -The mouse was touched with a blunt stick on each side of the body andthe reaction to the stimulus was observed. Scores indicate the following:3 = mouse reacted to the stimulus by turning head; 2 = mouse reacted slowly to stimulus; and 1 = mouse did not respond to the stimulus (Garcia et al., 1995).
Histological Examination
Three weeks after transplantation, mice were anaesthetized by intraperitoneal injection of chloral hydrate and trans-cardially perfused with 4% paraformaldehyde in phosphate buffer (0.1 M PB, pH 7.4. The spinal cord was dissected, andthe T8-L2 vertebral segment isolated and post-fixed in PFA for 2 h at 4°C.
Samples were transferred overnight into 30% sucrose in 0.1 M PB (phosphate buffer) at 4°C for cryoprotection,embedded in cryostat medium (Killik; Bio-Optica, Milan, Italy),and cut on the cryostat (Microm HM 550) in serial transverse 50 μm-thick sections, kept in PBS at 4°C or mounted onto gelatin-coated slides, to be processed for immunostaining. Before performing any further reaction, all sections were mounted in PBS, coverslipped and examined at a Nikon Eclipse E800 epifluorescence microscope under FITC- and DAPI-filter set to count surviving transplanted cells: in order to avoid double counting in consecutive sections, only cells contained entirely within the section were counted (partial cells, cut at the edges of the section, were not included in the counts). All morphological analyses were performed by examiners blinded to the group of the animal considered.
Immunofluorescence
For immunofluorescence, some sections (one every 600 μm) were immunolabelled with the following antibodies. After blocking aspecific binding sites 30 min RT with 0.3% Triton X-100 and 10% normal donkey serum (NDS; Sigma-Aldrich) in PBS pH 7.4, the sections were incubated with the following primary antibodies in the same solution at 4°C overnight (for the origin and specificity of the antibodies see also Boido et al., 2009): polyclonal anti-glial fibrillary acidic protein (GFAP; made in rabbit; 1:500; Dako Cytomation, Denmark); monoclonal anti-nestin (made in mouse; 1: 200; Chemicon International Inc, Temecula, CA, USA); monoclonal anti-microtubule associated protein-2 (MAP-2; made in mouse; 1:200; Chemicon); polyclonal anti-myelin basic protein (MBP; made in rabbit; 1: 200; Immunological Sciences); polyclonal anti-Ki67 (made in rabbit, 1: 400; Novocastra Laboratories Ltd., Newcastle, United Kingdom).
The sections were then washed in PBS and incubated in 1: 200 cyanine 3-conjugated secondary antibodies anti-rabbit or anti-mouse (Jackson Immuno Research Laboratories; 1200; West Grove, PA, USA).
The sections were examined with a Nikon Eclipse 90i epifluorescence microscopeand photographed by a Nikon DS-5Mc digital camera. Photomicrographs were manipulated and mounted in plates with the Photoshop CS2 software, with autocontrast enhancement. In order to check for double staining and to make 3D reconstructions, some preparations were examined also with a Leica TCS SP5 confocal laser scanning microscope (CLSM).
To evaluate astroglial activation(ICoE n = 7; ICo n = 5; IE n = 10; IS n = 8), GFAP immunoreactivity was analyzed. For semiquantitative analysis, we considered three sections, one at the lesion site, one 400 μm rostral, and another one 400 μm caudal. In particular, we quantified immunoreactivity in the dorsal horns (laminae I, II, III, IV, V) and in the ventral horns (IX). These areas were photographed using the Leica TCS SP5 at 40x. The percentage of the overall GFAP-positive area was quantified using the Scion Image software for Windows (freeware version of NIH image, Scion Corporation, Frederick, MD, USA), by two blind observers.
Analysis of the Lesion Volumes
For analyzing the lesion volume (ICoE n = 7; ICo n = 5; IEn = 10; IS n = 8), one 50 μm-thick section every two (total spinal cord segment 1600 μm-thick) was drawn at the computer using the Neurolucida software program (Microbrightfield Inc., VT, USA), and the volume obtained was analyzed with the Neuroexplorer program (Microbrightfield Inc.). The volume of the lesion was then expressed as a percentage of the total volume of the segment analyzed.
Luxol Fast Blue (LFB)Staining
One series of spinal cord sections was employed for analyzing the white matter sparing (ICoE n = 7; ICo n = 5; IE n = 9; IS n = 8). The sections were incubated in warm Luxol Fast Blue staining solution (0.1% in 95% ethanol) at 60°C for an hour. Afterwards, the sections were differentiated briefly in lithium carbonate solution (0.05%), rinsed with 70% ethanol, and then dehydrated in ethanol, cleared in xylene, and mountedfor microscopy. The spinal cord was drawn with the Neurolucida software program and reconstructed with the Neuroexplorer program: the spared volume of the white matter was expressed as a percentage of the total volume of the segment analyzed.
Statistics
Data are shown as mean ± SEM (standard error of the mean), and inter-group differences were statistically compared with one-way analysis of variance (ANOVA), followed by Bonferroni post-hoc test. Additionally, behavioral data were analyzed between 14th (last performance before treatment) and 35th day (last performance before sacrifice), using two-way ANOVA, followed by Bonferroni post-hoc test. Differences were considered significant when p ≤ 0.05.
Results
We report on the results of cotransplantation of NPs and MSCsin association or not with EE/exercise, in a compression injury model of spinal cord damage. Lesion size, inflammation and white matter sparing were analyzed and compared among groups, and correlated with the functional recovery.
Survival, Distribution and Differentiation of the NPs and MSCs
Two weeks after SCI, ICoE and ICo animals received a stem cell cocktail directly into the spinal lesion. Although the initial amount of transplanted NPs was higher than MSCs, after three weeks the ratio of surviving cells was totally reversed in both groups (Fig. 2a-b): in fact, counts of surviving cells revealed that many more MSCs (32% in ICoE and 13% in ICo) had survived than NPs (around1%). Performing immunofluorescence for Ki67, we identified some positive MSCs (< 0.5%), sign of a limited cellular proliferation (Fig 3a-d).
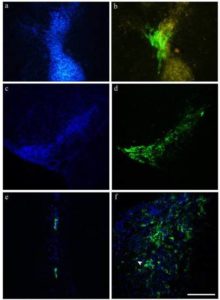
Figure 2- Survival and Distribution of NPs and MSCs
(a-b) MSCs, labeled in blue (a), can penetrate the lesion area, that appears yellow in the picture (b); on the other hand EGFP-NPs (b) preferentially surround the damaged area.
(c—d) When transplanted, MSCs and NPs remain organised in clusters. NPs display a heterogeneous morphology, from round profile to a fusiform neuronal-like phenotype. Moreover, MSCs and NPs are frequently overlapping: (e-f)occasionally it is possible to observe blue nuclei with green cytoplasm (possible sign of cell fusion).
Scale bar = 150 μm in a, b, 200 μm in c, d, and 100 μm in e, f.
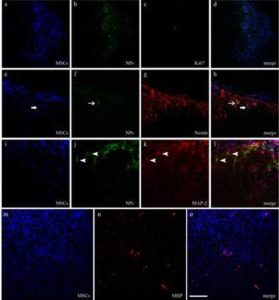
Figure 3 – Differentiation of NPs and MSCs
Differentiation of NPs and MSCs within host spinal cord is assessed respectively by colocalization of EGFP (green) or bisbenzimide (blue), and neural markers (labelled in red) at the CLSM.
(a-d) Immunoreactivity against Ki67 reveals a positive MSC. (e-h) Immunoreactivity against nestin highlights NP- and MSC-positive cells (arrows). (i-l) Immunoreactivity against MAP-2 shows some labelled NPs (arrow heads). (m-o)Some bisbenzimide-stained MSCs appear positive to MBP, a constituent of the myelin sheath of oligodendrocytes in the CNS.
Scale bar = 100 μm in a-l and 80 μm m-o.
Both cell types were distributed in the dorsal spinal cord (including the dorsal column and the dorsal horns), around the central canal and, to a small extent, into the ventral area (especially into the white matter).We detected cells especially close to the injection site: however, MSCs and NPs were respectively able to extend up to 3700 and 2000 μm, caudally and rostrally to the graft site.
In proximity of the lesion area, NPs preferentially surrounded it, whereas MSCs could penetrate it (Fig. 2a-b), as already observed in previous studies (Boido et al., 2009; Boido et al., 2011; Boido et al., 2012).
Relative to cell morphology, NPs were polymorphic, from round profile to a fusiform neuronal-like phenotype with several processes and ramifications (Fig. 2b, d-f). NPs showed the same polymorphic aspect when cultured in vitro (Boido et al., 2009). On the other hand, bisbenzimide labeling makes more difficult to identify theMSC morphology, that generally appears fibroblast-like both in vitro (Gunetti et al., 2012) and in vivo (Boido et al., 2012).
Both NPs and MSCs integrate into the host tissue, remaining close to each other (Fig. 2c-e). Occasionally we observed blue nuclei with green cytoplasm, suggesting that a cell fusion occurred (Fig. 2f).
We performed immunofluorescence reactions in order to detect differentiation of grafted cells. Both NPs and MSCs were positive to nestin, an early neuronal marker (Fig. 3e-h). Rarely NPs were positive to MAP-2, a marker of mature neurons (3i-l), whereas MSCs were positive to MBP, the major constituent of the myelin sheath (3m-o).On the contrary, both cells were negative for the astrocytic marker GFAP.
Analysis of the Lesion Volume
Three weeks after graft, we measured the size of the glial cyst using the Neurolucida software program: in fact the glial cyst is clearly recognizable for the increased natural staining of the lesion area. A 1600-µm spinal cord segment was serially reconstructed at the computer. We did not obtain statistically significant results, however a tendency was quite evident. The mean lesion volume, expressed as a percentage of the total volume of the segment analyzed,was 6.57% ± 0.29in ICoE mice, 6.93% ± 1.16 in ICo mice, 6.95% ± 0.85 in IE mice and 8, 65% ± 1.38 in IS mice, demonstrating a lesion volume reduction of approximately 24% (ICoE mice vs. IS mice) and 20% (ICo/IE mice vs. IS; Fig. 4).
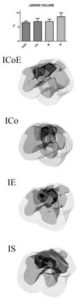
Figure 4 — Lesion Volume Analysis
The morphometric data were obtained using the Neurolucida software program. The graph summarizes the mean lesion volume, expressed as a percentage of the total volume of the segment analyzed.
White Matter Sparing
Using the Neurolucida software program we have calculated the percentage of white matter sparing following LFB staining: we analyzed a 4400µm spinal cord segment, evaluating every 8th section, for a total of 12 sections. Loss of LFB-stained areas was observed in all injured groups. Quantification of the spared white matter volume showed similar results in all groups, although it tendedto be inversely proportional to lesion volume measurement: ICoE group (49.20% ± 0.87), ICo group (48.33 ± 0.62), IE group (48.30% ± 0.94), IS group (47.30% ± 0.49; Fig. 5a).
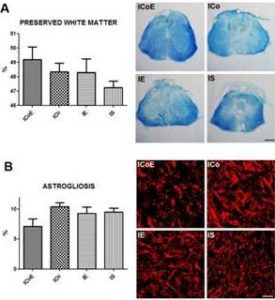
Figure 5 — White Matter Sparing and Astrogliosis
(a) On the left, we have quantified the spared white matter volume after SCI, using the Neurolucida software program. On the right, Luxol fast blue staining shows the preserved white matter. Scale bar = 300 μm.
(b) On the left semiquantitative analysis of GFAP immunoreactivity, relative dorsal and ventral horns at the lesion site; on the right pictures showing astrogliosis in ICoE, ICo, IE and IS groups. Scale bar = 30 μm.
Quantification of Astrogliosis
We have measured the GFAP immunoreactivity with a semiquantitative analysis, considering dorsal and ventral horns in three sections (at the lesion site, 400 μm rostrally and 400 μm caudally).
Basically, there were no important differences in the quantified areas among groups, however in the lesion section, the astrogliosis of ICoE was reduced (7.09% ± 1.31) compared to the other groups (ICo 10.43% ± 0.70, IE 9.30% ± 1.09, IS 9.52% ± 0.68; Fig 5b). These differences became more evident evaluating only the dorsal horns (the main site of lesion, since the weight is applied onto the dorsal spinal cord): indeed ICoE displayed a moderate astrogliosis (3.97% ± 1.58) in comparison to the other groups (ICo 12.12% ± 1.30, IE 8.44% ± 3.35, IS 7.44% ± 0.42).
Moreover, since the glial cyst is totally deprived of astrocytes, we have necessarily excluded some areas from the GFAP-quantification. In ICoE group, we discarded 4.14 ± 0.70 areas for animal, in ICo 4.20 ± 1.53, in IE 5.10 ± 0.70, and in IS 6.40 ± 2.00: this represents indirect data concerning the lesion dimension.
Behavioral Outcome
In order to assess the sensorimotor recovery after the treatments (graft and/or EE/exercise), mice underwent a battery of tests (BMS, foot-fault, hindlimb flexion and sensory tests; Fig. 6, column on the left). Additionally, we measured differences between performances at day 35 and day 14 (Fig. 6, column on the right), for understanding the effective improvements before (day 14) and after treatment (day 35).

Figure 6 – Behavioural Tests
Analysis of functional recovery of ICoE, ICo, IE and IS mice, studied with a battery of tests (Basso Mouse Scale, foot-fault, hindlimb flexion, sensory tests). The first column shows the scores obtained immediately before injury (day 0) and then 4-7-14-21-28-35 days post injury (where the values on 14th day are obtained immediately before graft and EE/exercise): two-way ANOVA was performed on performances between 14 and 35 day post injury. The scores are expressed as mean ± SEM. The second column displays the differences in ICoE, ICo, IE and IS performances between 35th and 14th day: positive values in the BMS and sensory test, and negative values in foot-fault test and hindlimb flexion test reflect improvements in the performance. One-way ANOVA was performed for comparing the relative improvements (*p ≤ 0.05, **p ≤ 0.01).
BMS -After SCI,all groups showed a slight increasing recovery of motor performance: however, the recovery further improved steadily until 35th day for ICoE, ICo and IE mice, whereas it did not change between the 14th day and sacrifice for IS group. On the 35th day post-injury, ICoE and ICo groups showed a similar performance with a final BMS score of 5.40 ± 0.42 and 5.48 ± 0.27 respectively, whereas IE mice displayed a score of 5 ± 0.72 and IS mice 4.18 ± 0.96: two-way ANOVA showed an extremely significant main effect for time (F4, 130 = 28.98, p <0.0001), in absence of significant differences for treatment (F3, 130 = 0.95, p = 0.4193)and interaction (F12, 130 = 0.12, p = 0.9999). Comparing performance between the 14th and 35th days, we documented respectively a 1.01-point improvement in the BMS score of ICoE mice, a 0.78-point improvement for ICo mice, a 0.65-point improvement for IE mice and a 0.00-point improvement for IS mice, respectively.
Foot-fault test —After treatment, at the 35th day, we counted 6.62 ± 1.50 errors for ICoE, 4.73 ± 1.27 for ICo, 12.53 ± 1.82 for IE, and 15.33± 2.53 for IS mice: two-way ANOVA uncovered an extremely significant main effect for treatment (F3, 130 = 7.28, p = 0.0001) and for time (F4,130 = 17.65, p < 0.0001), in absence of significant differences for interaction (F12, 130 = 0.94, p = 0.5050); post-hoc comparisons showed that at 35th day, the performance of ICoE and ICo mice was significant compared to IS mice (p < 0.05). Between the 14th and the 35thday, the percentage of improvement for ICoE was 22.06%, for ICo 12.89%, for IE 5.44%, and, on the contrary, IS group registered a percentage of worsening of 9.03% (one-way ANOVA ICoE vs. IS p < 0.01).
Hindlimb flexion test— At the 14th day, the performance score was comprised between 0.5 and 1.2 in all the groups. Afterwards, the treatments exerted positive effects: in fact at the 35thday, the score reduced respectively to 0.36 ± 0.14 in the ICoE group, 0.30 ± 0.20 in the ICo group, 0.75 ± 0.26 in the IE group, and 0.94 ± 0.32 in the IS group, demonstrating respectively a score improvement of about 0.57 (ICoE), 0.20 (ICo), 0.45 (IE) and 0.25 (IS). Two-way ANOVA showed an extremely significant main effect for treatment (F3, 130 = 6.49, p = 0.0004) and for time (F4, 130 = 9.38, p < 0.0001), without significant differences for interaction (F12, 130 = 0.59, p = 0.8487); additionally, post-hoc comparisons displayed that at the 21st day, the performance of ICo group was significant compared to IS mice (p < 0.05).
Sensory test — After lesion, every group showed a partial reduction in the sensory functions, that gradually increased until a nearly complete recovery at the 35th day. However, analyzing the scores between the 14th and 35th days, ICoE mice showed the highest score improvement (0.39), compared to ICo mice (0.30), IE mice (0.15)and IS mice (0.25). Two-way ANOVA yielded a significant main effect for treatment (F3, 130 = 3.32, p = 0.0220) and for time (F4, 130 = 9.38, p = 0.0004), without significant differences for interaction (F12, 130 = 0.57, p = 0.8638).
Discussion
We have previously shown beneficial results, in terms of induction of axonal sprouting, reduction of the lesion area, functional recovery, from treatment with NPs or MSCs inSCI: here, we have investigated the synergistic effects oftheir combined graft, i.e. cotransplantation, together with EE housing and locomotor exercise. We have observed that EE/exercise can lead to anatomical recovery in terms of lesion volume reduction and white matter sparing, but not fully correlated with a consequent functional improvement following SCI. Stem cell graft seems to better support such recovery. Moreover MSCs and NPs seem to reciprocally influence their proliferation and differentiation, probably by neurotrophic factor release.
Survival, Differentiation and Mutual Interactions between NPs and MSCs
Cotransplantation protocols have been tested only rarely, even thought hey can represent a valid approach to exploit the specific potential/characteristics of different stem cells (Pearse et al., 2007; Wang et al., 2010; Oh et al., 2011).
We have simultaneously grafted NPs and MSCs. NPs are embryonic stem cells including neuronal- and glial-restricted precursors (Mujtaba et al., 1999): when transplanted after SCI, NPs are able to integrate into host tissue and promote regeneration and repair (Lepore and Fischer, 2005). Moreover, we have already demonstrated that they stimulate the sprouting of serotonergic fibers following hemisection, resulting in a significant reorganization of spinal circuits and in a consequent functional improvement:after transplantation, NPs had very long axons and collateral branches with arbors(Boido et al., 2009). On the other hand, it has been demonstrated that MSCs can generate an environment highly permissive to cell survival and axonal growth, decreasing immunoreaction in the host, modulating inflammatory response to an insult and providing trophic factors for neurons and regenerating fibers (Azari et al., 2010; Salem and Thiemermann, 2010; Galindo et al., 2011; Garbossa et al., 2012).
At our experience, even though both cell types exerted positive effects when used separately after SCI, it should be noticed that, in both experimental conditions, i.e. hemisection or compression injury, and therapeutic time window (acute or delayed) we used, NPs displayed slightly better results compared to MSCs, especially relative to the ability in reducing the glial cyst. Here, we combined NPs and MSCs transplantation to exploit their specific characteristics. Even though in cotransplantation experiments cells from different populations are usually transplanted in a 1:1 ratio (Pearse et al., 2007; Puymirat et al., 2009; Wang et al., 2010), we used 2:1 NPs: MSCs. Our rationale consisted in using a lower amount of MSCs to create a permissive environment for NP survival/integration, modulating immune response and secreting soluble factors (Kassis et al., 2011; Garbossa et al., 2012).
Recently, electroporation and lipofection have been used for neurotrophic factor gene transfection, in order to enhance the intrinsic therapeutic efficacy of several stem cells (Rizvanov et al., 2008; Park et al., 2009; Fierro et al., 2011; Garbossa et al., 2012): though showing interesting results, they displaya limited transfection efficacy (less than 50%), consequently requiring a high initial number of cells (Griffin et al., 2010); moreover, gene therapy is not feasible in clinical practice for the risk of side effects. In order to circumvent this kind of problems, we decided to support the NP integration with the use of MSCs, acting as biologic mini pumps able to deliver trophic factors (BDNF, VEGF, NGF, NT3)and immunomodulatory molecules (Rossi and Keirstead, 2009; Azari et al., 2010; Garbossa et al., 2012).
However, three weeks after transplantation, the percentage of surviving NPs (around 2%) was comparable to that occurred in our previous experiments (Boido et al., 2011). Nevertheless, in the present paper we made the (delayed) transplant in a micro environment less permissive to cell survival then in the previous protocol, when transplantation occurred in the acute therapeutic time window.
The most surprising result consists in the impressive MSC survival.Previous studies on the interactions between MSCs and NSCs (neural stem cells) or ESCs (embryonic stem cells) in vitro and in vivo were more focused on neural cell rather than the MSC survival and differentiation (Kang et al., 2003; Lou et al., 2003; Matsuda et al., 2009; Puymirat et al., 2009; Oh et al., 2011). However, there is evidence that also neural precursors can secrete a wealth of trophic factors including BDNF, NT-3, NT-4/5, NGF, IGF and CNTF (ciliary neurotrophic factor), as demonstrated in vitro (Kamei et al., 2007; Hawryluk et al., 2011).In addition, differentiation of NPs led to a marked increase in their expression (Hawryluk et al., 2011), even though there are some origin-specific differences (Smith et al., 2003). Here, we found that transplanted NPs were positive to both nestin and MAP-2, respectively early and mature neuronal markers. In particular, Hawryluk and Coll. (2011) have compared the neurotrophin expression between cultured embryonic rat spinal cord NPs and bone marrow stromal cells, showing that NPs produce more CNTF and bFGF (basic fibroblast growth factor) compared to MSCs.When MSCs are cultured in presence of bFGF either in the culture medium or in the plate coating, the MSC proliferation increases, in particular depending on the bFGF in the medium (Hori et al., 2004). Notably, for the first time, we detected Ki67 positive MSCs. Therefore, it is possible that the strong MSC survival and proliferation here observed partially depend on the bFGF secretion by NPs.
Alexanian (2005) described the interactions occurring in vitro between NSCs and MSCs, demonstrating the importance of juxtacrine and paracrine effects combined with soluble factors released by NSCs to determinate MSC cell fate: in fact, co-culture of MSCs and NSCs induced the expression of nestin in MSCs (neurally-induced MSCs) when grown in presence of bFGF, and on the contrary it stimulated the MSC neuronal and glial morphology in its absence. In our study, for the first time, we observed nestin-positive MSCs: this suggests that the interactions with NPs have triggered the innate MSC plasticity, highlighting the extreme MSC immaturity and their undetermined fate (Garbossa et al., 2012).
With regard to the large number of bisbenzimide-positive MSC nuclei observed, we are aware that bisbenzimide could diffuse to the host tissue after transplantation of bisbenzimide-labelled cellsas a consequence of cell death, as suggested by some authors (Iwashita et al., 2000). On the other hand, the use of nuclear DNA staining as a stem cell marker in grafts is very common, in the absence of reports of die diffusion (Leiker et al., 2008; Assis et al., 2010; Jiang et al., 2011; Ling et al., 2011; Qin et al., 2011). However, in our experiments the mouse with the higher number of counted survived bisbenzimide-MSCs has also the higher number of NPs cells, and had the best behavioral performance, thus excluding a massive MSC death.
Finally, we have observed that also EE/exercise could influence the MSC, but not NP, survival: indeed the ICoE group showed an increased MSC number compared to ICo mice. We are going to discuss the effects induced by EE/exercise in the next paragraph.
Histological and Behavioral Effects of Graft and EE/Exercise
From the day of treatment (transplantation/saline), ICoE and IE animals were housed in environmental enriched cages in the presence of plastic tubing and running wheels. Depending on the object type, the literature reports that enriched the environment can determine sensory, cognitive or motor stimulation. As reviewed by van Praag and coll. (2000), EE exerts a variety of cellular, molecular and behavioral effects: enrichment increases cell survival, neurogenesis, dendritic branching and length, whereas exercise can induce cell proliferation and neuronal differentiation (Olson et al., 2006). EE increases the expression of several neurotrophic factors, as BDNF, GDNF, NGF and NT-3in the brain (Ickes et al., 2000), as well as exercise can increase FGF, BDNF and VEGF levels (Gobboand O’Mara, 2004; Tang et al., 2010). Some of these factors specifically influence the cell survival. For example, VEGF used in association with MSCs induces their survival, exerting also positive effects on angiogenesis, thus facilitating recovery after trauma (Rodrigues et al., 2010). Similarly, VEGF promotes NSC survival (Wada et al., 2006) and, NSCs over-expressing VEGF, transplanted in a murine stroke model, support neuroprotection, angiogenesis and functional recovery (Lee et al., 2007).BDNF and NGF are implicated in cell survival enhancement as well:as hypothesized by Olson (2006), EE/exercise probably activates the IP3 and MAPK pathways through a BDNF- and NGF-mediated mechanism, leading to cell proliferation (Choi et al., 2008) and survival (Bonni et al., 1999).
Therefore, also these observations can in part explain the great NP and, above all, MSC survival observed in this study compared to the previous ones (Boido et al., 2009; Boido et al., 2011), further partially enhanced by EE/exercise (see ICoE vs. ICo group results).
However, as concerns the histological/behavioral parameters, our results are quite controversial: IE group showed values regarding the lesion volume reduction and white matter sparing consistent with ICoE and ICo, but higher astrogliosis than ICoE, and limited functional recovery compared to ICoE and ICo mice. Indeed, white matter, quantified with LFB-staining, was best preserved in ICoE (49.20%), whereas its sparing was similar in ICo (48.33%) and IE (48.30%) mice,and worst in IS group (47.30%), although in the absence of statistical significance. Marques and Coll. (2010), obtaining similar results in a murine model of spinal cord compression after the injection of predifferentiated embryonic stem cells, proposed that these positive effects were due to the stem cell ability in secreting neurotrophic factors (BDNF, NT3, NGF), in this way minimizing the damage, determining remyelination and consequently improving functional repair.
In addition, we also obtained positive results in terms of reduction in lesion size, even though not statistically significant due to the large interindividual variability: morphometric analysis with Neurolucida software showed a lesion dimension reduction in all the treated groups compared to IS group. The present results appear reduced compared with those we previously obtained, when we administered the cells separately, and we observed a 63% reduction in NP-transplanted mice vs. the controls (Boido et al., 2011), and 32% reduction in MSC-transplanted mice vs. the controls (Boido et al., 2012). Nevertheless, the timing of cell administration (acute vs. delayed graft) was different: here, the spinal environment was less permissive and plastic, since all the degenerative and reactive processes were already established. This last approach has the most translational implications. Therefore, from this point of view, the results here obtained are certainly encouraging.
Finally, concerning GFAP-immunoreactivity, ICoE mice have a slightlylower GFAP expression (in particular in the dorsal horns), whereas the other groups display similar results. The role of astrocytes is still debated, since they can play a dual role after SCI, both harmful and beneficial (Renault-Mihara et al., 2008). In our experimental conditions, the transplanted cells seem able to modulate the GFAP expression when associated to EE/exercise, whereas graft or EE/exercise alone cannot induce the same effects.
However, these encouraging histological results do not fully correlate with the behavioral data: at the 35th day after SCI, ICoE and ICo mice showed the best performance in all the tests (except in the sensory test for ICo); also IE group obtained some improvements, but less remarkable than ICoE and ICo; finally IS mice showed the worst outcome, characterized by a weak physiological improvement or, in the case of foot-fault test, even worsening.
Other papers concerning EE/exercise have described such unusual results: for example, Lankhorst and Coll. (2001) reported locomotor recovery in contused trained rats, without observing differences among the experimental groups in electrophysiological parameters and nor in the sparing of white matter. The authors suggested the need of a more controlled exercise training, in order to exploit it as a potential additive approach. Therefore, we can here hypothesize that EE/exercise could only in part represent a benefit as a therapeutic approach.
Considerations on EE/Exercise Effects
Based on our observations, this study suggests that the beneficial effects obtained in particular in the behavioral tests are mainly supported by stem cell graft rather than by EE/exercise. Indeed NPs and MSCs, integrating into the host tissue and secreting neurotrophic factors and immunomodulatory molecules, can generate a favorable micro environment to support surviving host motoneurons and promote axonal regeneration and CNS repair (Garbossa et al., 2012)..
On the other hand, the recovery led by EE/exercise appears modest. Several authors consider EE/exerciseto be able to determine cell survival, proliferation, axonal growth and oligodendrogenesis. For example, Buchhold and Coll (2007) demonstrated that in both aged and young rats the environmental enrichment improved the rate and extent of recovery following stroke.
It is evident that in our experiments, EE/exercise partially contributes inameliorating the histological and behavioral parameters, but clearly its effect is not comparable to that of ICoE and ICo (above all in the sensorimotor tests).
Indeed, some authors sustain that recovery after SCI generally depends on the type and intensity of training (Basso, 2011). As reviewed by Battistuzzo and coworkers (2012), in experimental SCI conditions, the most used types of intervention are represented by treadmill training (33%), body weight-supported treadmill training (BWSTT; 17%), voluntary wheel running (17%), swimming (17%) and environmental enrichment (17%), but regardless of lesion type, treadmill training seems the most effective. However, also a successful training as BWSTT for contused rats become inefficient (in terms of functional recovery, muscle atrophy and axonal sprouting), when applied in a discontinuing way (Singh et al., 2011). Additionally, some authors refer that for chronic lesions the greater improvements are obtained when BWSTT is associated to functional electrical stimulation (Morawietz and Moffa, 2013). Therefore, it is clear that a specific training is critical to induce a consistent recovery after incomplete SCI.
However in literature, the degree of recovery observed following forced exercise results highly variable and the lack of consistency among labs suggests that exercise can be only modestly effective in improving the locomotion after incomplete SCI (Jakeman et al., 2011). Indeed, one of the main problems is to identify the correct timing, type and intensity of training required for optimal recovery. Some authors hypothesize that “any forced exercise that can significantly increase the cardiovascular output at acute or sub-acute post-SCI time points, can bring about increased extravasation of macromolecules into the spinal cord parenchyma, potentially altering the progression of the secondary injury” (Smith et al., 2009). In our experiments, the exercise is mainly free, except for 5 min/day when we forced the ICoE and IE mice on wheels, therefore we can probably exclude such hypothesis. However, also spontaneous exercise implies some problems: in fact, although rodents show an innate motivation, in case of SCI the response is not univocal: additionally it is difficult to quantify the time spent by the animal in physical activity, an aspect depending on its sedentary or active nature, and therefore lacking standardization (Jakeman et al., 2011).
Conclusion
In this paper, we have investigated the synergistic effects of NP and MSC cotransplantation, in association with EE housing and locomotor exercise.
To summarize, we can conclude that stem cells cotransplantation can assure a remarkable anatomical and functional recovery following SCI, and such recovery is only partially boosted by EE/exercise. The observed improvements are probably due to the stem cell ability insecreting neurotrophic factors (BDNF, VEGF, NGF, NT-3) and immunomodulatory molecules, in integrating into the host circuits, bridging the lesion cavity, promoting neuronal and axonal regeneration (Garbossa et al., 2012); on the other hand, the literature reports that EE/exercise might induce several beneficial effects, such as increase of cell survival and synaptogenesis, neuroprotection, angiogenesis, enhancement oftrophic factor expression (Will et al., 2004).
Our results highlighted that EE/exercise can produce some limited histological improvements in absence of correlated behavioral recovery, and that the main observed benefits are probably due to stem cell action. However, since recovery after SCI generally depends on timing, type and intensity of training, additional studies are needed for setting the better exercise parameters in order to enhance the restorative effects and the functional recovery.
Acknowledgements
Supported by grants from Girotondo Onlus, FAIP (Federazione delle Associazioni Italiane Paraplegici).
(adsbygoogle = window.adsbygoogle || []).push({});
References
Alexanian, A. R. (2005). “Neural Stem Cells Induce Bone-Marrow-Derived Mesenchymal Stem Cells to Generate Neural Stem-Like Cells via Juxtacrine and Paracrine Interactions,” Experimental Cell Research, 310 (2) 383-391.
Publisher – Google Scholar
Assis, A. C. M., Carvalho, J. L, Jacoby, B. A., Ferreira, R. L., Castanheira, P., Diniz, S. O., Cardoso, V. N., Goes, A. M. & Ferreira, A. J. (2010). “Time-Dependent Migration of Systemically Delivered Bone Marrow Mesenchymal Stem Cells to the Infarcted Heart,” Cell Transplantation, 19 (2) 219-230.
Publisher – Google Scholar
Azari, M. F., Mathias, L., Ozturk, E., Cram, D. S., Boyd, R. L. & Petratos, S. (2010). “Mesenchymal Stem Cells for Treatment of CNS Injury,” Current Neuropharmacology, 8 (4) 316-323.
Publisher – Google Scholar
Basso, D. M. (2011). “Invited Commentary,” Physical Therapy, 91 (1) 60-62.
Publisher
Basso, D. M., Fisher, L. C., Anderson, A. J., Jakeman, L. B.., Mctigue, D. M. & Popovich, P. G. (2006). “Basso Mouse Scale for Locomotion Detects Differences in Recovery after Spinal Cord Injury in Five Common Mouse Strains,” Journal of Neurotrauma, 23 (5) 635-659.
Publisher – Google Scholar
Battistuzzo, C. R., Callister, R. J., Callister, R. & Galea, M. P. (2012). “A Systematic Review of Exercise Training to Promote Locomotor Recovery in Animal Models of Spinal Cord Injury,” Journal of Neurotrauma, 29 (8) 1600-1613.
Publisher – Google Scholar
Berrocal, Y., Pearse, D. D., Singh, A., Andrade, C. M., Mcbroom, J. S., Puentes, R. & Eaton, M. J. (2007). “Social and Environmental Enrichment Improves Sensory and Motor Recovery after Severe Contusive Spinal Cord Injury in the Rat,”Journal of Neurotrauma, 24 (11) 1761-1772.
Publisher – Google Scholar
Boido, M., Garbossa, D., Fontanella, M., Ducati, A. & Vercelli, A. (2012). “Mesenchymal Stem Cell Transplantation Reduces Glial Cyst and Improves Functional Outcome after Spinal Cord Compression,” World Neurosurgery.
Publisher – Google Scholar
Boido, M., Garbossa, D. & Vercelli, A. (2011). “Early Graft of Neural Precursors in Spinal Cord Compression Reduces Glial Cyst and Improves Function,” Journal of Neurosurgery. Spine, 15 (1) 97-106.
Publisher – Google Scholar
Boido, M., Rupa, R., Garbossa, D., Fontanella, M., Ducati, A. & Vercelli, A. (2009). “Embryonic and Adult Stem Cells Promote Raphespinal Axon Outgrowth and Improve Functional Outcome Following Spinal Hemisection in Mice,” The European Journal of Neuroscience, 30 (5) 833-846.
Publisher – Google Scholar
Bonni, A., Brunet, A., West, A. E., Datta, S. R., Takasu, M. A. & Greenberg, M. E. (1999). “Cell Survival Promoted by the Ras-MAPK Signaling Pathway by Transcription-Dependent and -Independent Mechanisms,” Science, 286 (5443) 1358-1362.
Publisher – Google Scholar
Buchhold, B., Mogoanta, L., Suofu, Y., Hamm, A., Walker, L., Kessler, C. H. & Popa-Wagner, A. (2007). “Environmental Enrichment Improves Functional and Neuropathological Indices Following Stroke in Young and Aged Rats,” Restorative Neurology and Neuroscience, 25 (5-6) 467-484.
Publisher – Google Scholar
Castanheira, P., Torquetti, L. T., Magalhãs, D. R. S., Nehemy, M. B. & Goes, A. M. (2009). “DAPI Diffusion after Intravitreal Injection of Mesenchymal Stem Cells in the Injured Retina of Rats,” Cell Transplantation, 18 (4) 423-431.
Publisher – Google Scholar
Choi, S. C., Kim, S. J., Choi, J. H., Park, C. Y., Shim, W. J. & Lim, D. S. (2008). “Fibroblast Growth Factor-2 and -4 Promote the Proliferation of Bone Marrow Mesenchymal Stem Cells by the Activation of the PI3K-Akt and ERK1/2 Signaling Pathways,” Stem Cells and Development, 17 (4) 725-736.
Publisher – Google Scholar
Döbrössy, M. D. & Dunnett, S. B. (2006). “Morphological and Cellular Changes within Embryonic Striatal Grafts Associated with Enriched Environment and Involuntary Exercise,” European Journal of Neuroscience, 24 (11) 3223-3233.
Publisher – Google Scholar
Engesser-Cesar, C., Ichiyama, R. M., Nefas, A. L., Hill, M. A., Edgerton, V. R., Cotman, C. W. & Anderson, A. J. (2007). “Wheel Running Following Spinal Cord Injury Improves Locomotor Recovery and Stimulates Serotonergic Fiber Growth,” Theeuropean Journal of Neuroscience, 25 (7) 1931-1939.
Publisher – Google Scholar
Farooque, M. (2000). “Spinal Cord Compression Injury in the Mouse: Presentation of a Model Including Assessment of Motor Dysfunction,” Acta Neuropathologica, 100 (1) 13-22.
Publisher – Google Scholar
Fierro, F. A., Kalomoiris, S., Sondergaard, C. S. & Nolta, J. A. (2011). “Effects on Proliferation and Differentiation of Multipotent Bone Marrow Stromal Cells Engineered to Express Growth Factors for Combined Cell and Gene Therapy,”Stem Cells, 29 (11) 1727-1737.
Publisher – Google Scholar
Fischer, F. R. & Peduzzi, J. D. (2007). “Functional Recovery in Rats with Chronic Spinal Cord Injuries after Exposure to an Enriched Environment,” The Journal of Spinal Cord Medicine, 30 (2) 147-155.
Publisher – Google Scholar
Galindo, L. T., Filippo, T. R., Semedo, P., Ariza, C. B., Moreira, C. M., Camara, N. O. & Porcionatto, M. A. (2011). “Mesenchymal Stem Cell Therapy Modulates the Inflammatory Response in Experimental Traumatic Brain Injury,”Neurology Research International, 2011:564089.
Publisher – Google Scholar
Garbossa, D., Boido, M., Fontanella, M., Fronda, C., Ducati, A. & Vercelli, A. (2012). “Recent Therapeutic Strategies for Spinal Cord Injury Treatment: Possible Role of Stem Cells,” Neurosurgical Review, 35 (3) 293-311.
Publisher – Google Scholar
Garcia, J. H., Wagner, S., Liu, K. F. & Hu, X. J. (1995). “Neurological Deficit and Extent of Neuronal Necrosis Attributable to Middle Cerebral Artery Occlusion in Rats,” Stroke; A Journal of Cerebral Circulation, 26 (4) 627-634.
Publisher – Google Scholar
Gobbo, O. L. & O’Mara, S. M. (2004). “Impact of Enriched-Environment Housing on Brain-Derived Neurotrophic Factor and on Cognitive Performance after a Transient Global Ischemia,” Behavioural Brain Research, 152 (2) 231-241.
Publisher – Google Scholar
Gómez-Pinilla, F., Dao, L. & So, V. (1997). “Physical Exercise Induces FGF-2 and Its Mrna in the Hippocampus,” Brain Research, 764 (1-2) 1-8.
Publisher – Google Scholar
Griffin, M., Greiser, U., Barry, F., O’Brien, T. & Ritter, T. (2010). “Genetically Modified Mesenchymal Stem Cells and Their Clinical Potential in Acute Cardiovascular Disease,” Discovery Medicine, 9 (46) 219-223.
Publisher – Google Scholar
Gunetti, M., Tomasi, S., Giammò, A., Boido, M., Rustichelli, D., Mareschi, K., Errichiello, E., Parola, M., Ferrero, I., Fagioli, F., Vercelli, A. & Carone, R. (2012). “Myogenic Potential of Whole Bone Marrow Mesenchymal Stem Cells in Vitro and in Vivo for Usage in Urinary Incontinence,” PloS One, 7 (9) E45538.
Publisher – Google Scholar
Hawryluk, G. W. J., Mothe, A. J., Chamankhah, M., Wang, J., Tator, C. & Fehlings, M. G. (2011). “In Vitro Characterization of Trophic Factor Expression in Neural Precursor Cells,” Stem Cells and Development.,21(3):432-47.
Publisher – Google Scholar
Hicks, A. U., Hewlett, K., Windle, V., Chernenko, G., Ploughman, M., Jolkkonen, J., Weiss, S. & Corbett, D. (2007). “Enriched Environment Enhances Transplanted Subventricular Zone Stem Cell Migration and Functional Recovery after Stroke,” Neuroscience, 146 (1) 31-40.
Publisher – Google Scholar
Hori, Y., Inoue, S., Hirano, Y. & Tabata, Y. (2004). “Effect of Culture Substrates and Fibroblast Growth Factor Addition on the Proliferation and Differentiation of Rat Bone Marrow Stromal Cells,” Tissue Engineering,10 (7-8) 995-1005.
Publisher – Google Scholar
Ickes, B. R., Pham, T. M., Sanders, L. A., Albeck, D. S., Mohammed, A. H. & Andgranholm. A. C. (2000). “Long-Term Environmental Enrichment Leads to Regional Increases in Neurotrophin Levels in Rat Brain,” Experimental Neurology,164 (1) 45-52.
Publisher – Google Scholar
Iwashita, Y., Crang, A. J. & Blakemore, W. F. (2000). “Redistribution of Bisbenzimide Hoechst 33342 from Transplanted Cells to Host Cells,” Neuroreport, 11 (5) 1013-1016.
Publisher – Google Scholar
Jakeman, L. B., Hoschouer, E. L. & Basso, D. M. (2011). “Injured Mice at the Gym: Review, Results and Considerations for Combining Chondroitinase and Locomotor Exercise to Enhance Recovery after Spinal Cord Injury,” Brain Research Bulletin, 84 (4-5) 317-326.
Publisher – Google Scholar
Jiang, Y., Lv, H., Huang, S., Tan, H., Zhang, Y. & Li, H. (2011). “Bone Marrow Mesenchymal Stem Cells Can Improve the Motor Function of a Huntington’s Disease Rat Model,” Neurological Research, 33 (3) 331-337.
Publisher – Google Scholar
Johansson, B. B. (1996). “Functional Outcome in Rats Transferred to an Enriched Environment 15 Days after Focal Brain Ischemia,” Stroke; A Journal of Cerebral Circualtion, 27 (2) 324-326.
Publisher – Google Scholar
Kamei, N., Tanaka, N., Oishi, Y., Hamasaki, T., Nakanishi, K., Sakai, N. & Ochi, M. (2007). “BDNF, NT-3, and NGF Released from Transplanted Neural Progenitor Cells Promote Corticospinal Axon Growth in Organotypiccocultures,”Spine (Phila Pa 1976), 32 (12) 1272-1278.
Publisher – Google Scholar
Kang, S. K., Jun, E. S., Bae, Y. C. & Jung, J. S. (2003). “Interactions between Human Adipose Stromal Cells and Mouse Neural Stem Cells in Vitro,” Brain Research. Developmental Brain Research, 145 (1) 141-149.
Publisher – Google Scholar
Kassis, I., Vaknin-Dembinsky, A. & Karussis, D. (2011). “Bone Marrow Mesenchymal Stem Cells: Agents of Immunomodulation and Neuroprotection,” Current Stem Cell Research and Therapy, 6 (1) 63-68.
Publisher – Google Scholar
Kobilo, T., Liu, Q. R., Gandhi, K., Mughal, M., Shaham, Y. & Van Praag, H. (2011). “Running is the Neurogenic and Neurotrophic Stimulus in Environmental Enrichment,” Learning and Memory,18 (9) 605-609.
Publisher – Google Scholar
Lankhorst, A. J., Terlaak, M. P., Van Laar, T. J., Van Meeteren, N. L., De Groot, J. C., Schrama, L. H., Hamers, F. P. & Gispen, W. H. (2001). “Effects of Enriched Housing on Functional Recovery after Spinal Cord Contusive Injury in the Adult Rat,” Journal of Neurotrauma, 18 (2) 203-215.
Publisher – Google Scholar
Lee, H. J., Kim, K. S., Park, I. H. & Kim, S. U. (2007). “Human Neural Stem Cells Over-Expressing VEGF Provide Neuroprotection, Angiogenesis and Functional Recovery in Mouse Stroke Model,” PloS One, 2 (1) E156.
Publisher – Google Scholar
Leiker, M., Suzuki, G., Iyer, V. S., Canty, J. M. Jr & Lee, T. (2008). “Assessment of a Nuclear Affinity Labeling Method for Tracking Implanted Mesenchymal Stem Cells,” Cell Transplantation, 17 (8) 911-922.
Publisher – Google Scholar
Lepore, A. C. & Fischer, I. (2005). “Lineage-Restricted Neural Precursors Survive, Migrate, and Differentiate Following Transplantation into the Injured Adult Spinal Cord,” Experimental Neurology, 194 (1) 230— 242.
Publisher – Google Scholar
Lewis, M. H. (2004). “Environmental Complexity and Central Nervous System Development and Function,” Mental Retardation and Developmental Disabilities Research Reviews, 10 (2) 91-95.
Publisher – Google Scholar
Ling, S. K., Wang, R., Dai, Z. Q., Nie, J. L., Wang, H. H., Tan, Y. J., Cao, H. Q., Huang, Z. M., Wan, Y. M. & Li, Y. H. (2011). “Pretreatment of Rat Bone Marrow Mesenchymal Stem Cells with a Combination of Hypergravity and 5-Azacytidine Enhances Therapeutic Efficacy for Myocardial Infarction,” Biotechnology Progress., 27 (2) 473-482.
Publisher – Google Scholar
Lou, S., Gu, P., Chen, F., He, C., Wang, M. & Lu, C. (2003). “The Effect of Bone Marrow Stromal Cells on Neuronal Differentiation of Mesencephalic Neural Stem Cells in Sprague-Dawley Rats,” Brain Research, 968 (1) 114-121.
Publisher – Google Scholar
Marques, S. A., Almeida, F. M., Fernandes, A. M., Dos Santos Souza, C., Cadilhe, D. V., Rehen, S. K. & Martinez, A. M. (2010). “Predifferentiated Embryonic Stem Cells Promote Functional Recovery after Spinal Cord Compressive Injury,”Brain Research, 1349 115-128.
Publisher – Google Scholar
Morawietz, C. & Moffat, F. (2013). “Effects of Locomotor Training after Incomplete Spinal Cord Injury: A Systematic Review,” Archivesof Physical Medicine and Rehabilitation.
Publisher – Google Scholar
Mujtaba, T., Piper, D. R., Kalyani, A., Groves, A. K., Lucero, M. T. & Rao, M. S. (1999). “Lineage-Restricted Neural Precursors Can be Isolated from Both the Mouse Neural Tube and Cultured ES Cells,” Developmental Biology, 214 (1) 113—127.
Publisher – Google Scholar
Nithianantharajah, J. & Hannan, A. J. (2006). “Enriched Environments, Experience-Dependent Plasticity and Disorders of the Nervous System,” Nature Reviews. Neuroscience, 7 (9) 697-709.
Publisher – Google Scholar
Oh, J. S., Kim, K. N., An, S. S., Pennant, W. A., Kim, H. J., Gwak, S. J., Yoon Do, H., Lim, M. H., Choi, B. H. & Ha, Y. (2011). “Cotransplantation of Mouse Neural Stem Cells (mNSCs) with Adipose Tissue-Derived Mesenchymal Stem Cells Improves mNSC Survival in a Rat Spinal Cord Injury Model,” Cell Transplantation, 20 (6) 837-849.
Publisher – Google Scholar
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997). “Green Mice’ as a Source of Ubiquitous Green Cells,” FEBS Letters, 407 (3) 313-319.
Publisher – Google Scholar
Okano, H. (2002). “Stem Cell Biology of the Central Nervous System,” Journal of Neuroscience Research, 69 (6) 698-707.
Publisher – Google Scholar
Olson, A. K., Eadie, B. D., Ernst, C. & Christie, B. R. (2006). “Environmental Enrichment and Voluntary Exercise Massively Increase Neurogenesis in the Adult Hippocampus via Dissociable Pathways,” Hippocampus, 16 (3) 250-260.
Publisher – Google Scholar
Park, S., Kim, H. T., Yun, S., Kim, I. S., Lee, J., Lee, I. S. & Park, K. I. (2009). “Growth Factor-Expressing Human Neural Progenitor Cell Grafts Protect Motor Neurons but Do Not Ameliorate Motor Performance and Survival in ALS Mice,” Experimental and Molecular Medicine, 41 (7) 487-500.
Publisher – Google Scholar
Pearse, D. D., Sanchez, A. R., Pereira, F. C., Andrade, C. M., Puzis, R., Pressman, Y., Golden, K., Kitay, B. M., Blits, B., Wood, P. M. & Bunge, M. B. (2007). “Transplantation of Schwann Cells and/or Olfactory Ensheathing Glia into the Contused Spinal Cord: Survival, Migration, Axon Association, and Functional Recovery,” Glia, 55 (9) 976-1000.
Publisher – Google Scholar
Pitsikas, N., Brambilla, A., Besozzi, C., Bonali, P., Fodritto, F., Grippa, N., Scandroglio, A. & Borsini, F. (2001). “Effects of Cerestat and NBQX on Functional and Morphological Outcomes in Rat Focal Cerebral Ischemia,” Pharmacology, Biochemistry and Behaviour, 68 (3) 443-447.
Publisher – Google Scholar
Puymirat, E., Geha, R., Tomescot, A., Bellamy, V., Larghero, J., Trinquart, L., Bruneval, P., Desnos, M., Hagège, A., Pucéat, M. & Menasché, P. (2009). “Can Mesenchymal Stem Cells Induce Tolerance to Cotransplanted Human Embryonic Stem Cells?,” Molecular Therapy: The Journal of the American Society of Gene Therapy, 17 (1) 176-182.
Publisher – Google Scholar
Qin, Z. H., Qu, J. M., Xu, J. F., Zhang, J., Summah, H., Sai-Yin, H. X., Chen, C. M. & Yu, L. (2011). “Intrapleural Delivery of Mesenchymal Stem Cells: A Novel Potential Treatment for Pleural Diseases,” Acta Pharmacologicasinica,32 (5) 581-590.
Publisher – Google Scholar
Renault-Mihara, F., Okada, S., Shitaba, S., Nakamura, M., Toyama, Y. & Okano, H. (2008). “Spinal Cord Injury: Emerging Beneficial Role of Reactive Astrocytes’ Migration,” The International Journal of Biochemistry and Cell Biology.40 (9) 1649-1653.
Publisher – Google Scholar
Rizvanov, A. A., Kiyasov, A. P., Gaziziov, I. M., Yilmaz, T. S., Kaligin, M. S., Andreeva, D. I., Shafigullina, A. K., Guseva, D. S., Kiselev, S. L., Matin, K., Palotás, A. & Islamov, R. R. (2008). “Human Umbilical Cord Blood Cells Transfected with VEGF and L(1)CAM Do Not Differentiate into Neurons but Transform into Vascular Endothelial Cells and Secrete Neuro-Trophic Factors to Support Neuro-Genesis-A Novel Approach in Stem Cell Therapy,” Neurochemistry International, 53 (6-8) 389-394.
Publisher – Google Scholar
Rodrigues, M., Griffith, L. G. & Wells, A. (2010). “Growth Factor Regulation of Proliferation and Survival of Multipotential Stromal Cells,” Stem Cell Research and Therapy,1 (4) 32.
Publisher – Google Scholar
Rosenzweig, M. R. & Bennett, E. L. (1996). “Psychobiology of Plasticity: Effects of Training and Experience on Brain and Behavior,” Behavioural Brain Research, 78 (1) 57-65.
Publisher – Google Scholar
Rossi, S. L. & Keirstead, H. S. (2009). “Stem Cells and Spinal Cord Regeneration,” Current Opinion in Biotechnology, 20 (5) 552-562.
Publisher – Google Scholar
Salem, H. K. & Thiemermann, C. (2010). “Mesenchymal Stromal Cells: Current Understanding and Clinical Status,”Stem Cells, 28 (3) 585-596.
Publisher – Google Scholar
Schrepfer, S., Deuse, T., Lange, C., Katzenberg, R., Reichenspurner, H., Robbins, R. C. & Pelletier, M. P. (2007). “Simplified Protocol to Isolate, Purify, and Culture Expand Mesenchymal Stem Cells,” Stem Cells and Development, 16 (1) 105-107.
Publisher – Google Scholar
Singh, A., Balasubramanian, S., Murray, M., Lemay, M. & Houle, J. (2011). “Role of Spared Pathways in Locomotor Recovery after Body-Weight-Supported Treadmill Training in Contused Rats,” Journal of Neurotrauma, 28 (12) 2405-2416.
Publisher – Google Scholar
Smith, R., Bagga, V. & Fricker-Gates, R. A. (2003). “Embryonic Neural Progenitor Cells: The Effects of Species, Region, and Culture Conditions on Long-Term Proliferation and Neuronal Differentiation,” Journal of Hematotherapy and Stem Cell Research, 12 (6) 713—725.
Publisher – Google Scholar
Smith, R. R., Brown, E. H., Shum-Siu, A., Whelan, A., Burke, D. A., Benton, R. L. & Magnuson, D. S. (2009). “Swim Training Initiated Acutely after Spinal Cord Injury is Ineffective and Induces Extravasation in and around the Epicenter,”Journal of Neurotrauma, 26 (7) 1017-1027.
Publisher – Google Scholar
Takamatsu, H., Tatsumi, M., Nitta, S., Ichise, R., Muramatsu, K., Iida, M., Nishimura, S. & Umemura, K. (2002). “Time Courses of Progress to the Chronic Stage of Middle Cerebral Artery Occlusion Models in Rats,” Experimental Brain Research, 146 (1) 95-102.
Publisher – Google Scholar
Tang, K., Xia, F. C., Wagner, P. D. & Breen, E. C. (2010). “Exercise-Induced VEGF Transcriptional Activation in Brain, Lung and Skeletal Muscle,” Respiratory Physiology Neurobiology, 170 (1) 16-22.
Publisher – Google Scholar
Thuret, S., Moon, L. D. F. & Gage, F. H. (2006). “Therapeutic Interventions after Spinal Cord Injury,” Nature Review. Neuroscience, 7(8):628-43.
Publisher – Google Scholar
Tropel, P., Noël, D., Platet, N., Legrand, P., Benabid, A. L. & Berger, F. (2004). “Isolation and Characterisation of Mesenchymal Stem Cells from Adult Mouse Bone Marrow,” Experimental Cell Research, 295 (2) 395-406.
Publisher – Google Scholar
Van Praag, H., Kempermann, G. & Gage, F. H. (2000). “Neural Consequences of Environmental Enrichment,” Nature Reviews. Neuroscience, 1 (3) 191—198.
Publisher – Google Scholar
Van Tendeloo, V. F. I., Van Broeckhoven, C. & Berneman, Z. N. (2001). “Gene Therapy: Principles and Applications to Hematopoietic Cells,” Leukemia, 15 (4) 523-544.
Publisher – Google Scholar
Wada, T., Haigh, J. J., Ema, M., Hitoshi, S., Chaddah, R., Rossant, J., Nagy, A. & Van Der Kooy, D. (2006). “Vascular Endothelial Growth Factor Directly Inhibits Primitive Neural Stem Cell Survival but Promotes Definitive Neural Stem Cell Survival,” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26 (25) 6803-6812.
Publisher – Google Scholar
Wang, G., Ao, Q., Gong, K., Zuo, H., Gong, Y. & Zhang, X. (2010). “Synergistic Effect of Neural Stem Cells and Olfactory Ensheathing Cells on Repair of Adult Rat Spinal Cord Injury,” Cell Transplantation, 19 (10) 1325-1337.
Publisher – Google Scholar
Will, B., Galani, R., Kelche, C. & Rosenzweig, M. R. (2004). “Recovery from Brain Injury in Animals: Relative Efficacy of Environmental Enrichment, Physical Exercise or Formal Training (1990-2002),” Progress in Neurobiology, 72 (3) 167-182.
Publisher – Google Scholar
Wolf, S. A., Kronenberg, G., Lehmann, K., Blankenship, A., Overall, R., Staufenbiel, M. & Kempermann, G. (2006). “Cognitive and Physical Activity Differently Modulate Disease Progression in the Amyloid Precursor Protein (APP)-23 Model of Alzheimer’s Disease,” Biological Psychiatry, 60 (12) 1314-1323.
Publisher – Google Scholar
Zhang, L., Schallert, T., Zhang, Z. G., Jiang, Q., Arniego, P., Li, Q., Lu, M. & Chopp, M. (2002). “A Test for Detecting Long-Term Sensorimotor Dysfunction in the Mouse after Focal Cerebral Ischemia,” Journal of Neuroscience Methods,117 (2) 207-214.
Publisher – Google Scholar








