Introduction
Complete or partial removable dental prostheses unadapted and poorly cleaned may induce some pathological changes on the mucosa of the oral cavity (Mandali et al., 2011). Prosthetic stomatitis by infestation of Candida species is the most frequent and it is characterized by moderate or severe mucosal inflammation underneath the denture base. Candida albicans is not only able to adhere to the mucous surfaces, but also to colonize the acrylic resins of the dental prosthesis. Both the plaque accumulated on the denture and the poor oral hygiene contribute to the virulence of Candida promoting a clinical picture of Candida-associated denture stomatitis (Salerno et al., 2011). Classic study has shown that this oral inflamation may occur on the maxilla and mandible; however, it is more often associated with the maxilla, sometimes found under maxilla partial dentures, but only rarely beneath mandibular dentures (Wilson, 1998). After colonization and adhesion of Candida species to the epithelial surface, the subsequent mucosal lesion is due to tissue destruction by potent proteolytic enzymes or toxins and an inflammatory response to Candida antigens.
There are other factors that favour the develop of oral candidiasis, such as denture base fit, metabolic disorder, patient’s age, mucosa conditions, epithelial changes, poor diet, appropriate denture hygiene, xerostomia and salivary flow (Mandali et al., 2011; Wilson, 1998; Budtz-Jorgensen, 1990; Pires et al., 2002; Torres et al., 2002 and Gendrau & Loewy, 2011). Study had shown that oral infestation by Candida species occurs commonly in the presence of denture that creates an environment that favours the localization and development of potentially virulent organisms (Pusateri et al., 2009). In addition, persistent denture stomatitis is due to immature antimicrobial host defenses, acquired suppression of immune defense mechanisms, or changes of the environmental conditions of the oral cavity (Budtz-Jorgensen, 1990).
Various drugs have been used for the treatment of denture stomatitis, and systemic antifungal therapies are also commonly indicated. However, previous study has reported the appearance of resistant microorganisms during long-term treatments or prophylactic. Although these drugs are effective in relieving the signs and symptoms of denture stomatitis,Candida biofilm-associated infections are difficult to treat and to fully eradicate with standard antifungal therapy (Ramage & Lopez-Ribot, 2005). Topical antimycotic treatment is used to prevent spread of the infection that occurs on the fitting denture surface by Candida species; however, recurrences are frequent if the local or the systemic predisposing conditions are not corrected (Budtz-Jorgensen, 1990). Candida associated denture stomatitis has also been associated with a lack of denture hygiene (Webb et al., 2005).
An important predisposing factor for stomatitis associated with Candida is the permanence of high levels of microorganisms in saliva (Pires et al., 2002). By this reason, a proper oral hygiene and efficient denture cleaning must be continuous for the bacterial biofilm control present on the denture base and oral soft tissue (Salerno et al., 2011). However, preventive post-insertion to adjust defective dentures and providing information on cleaning methods and instructions to maintain oral tissues healthy is also important in relation to oral care (Coelho et al., 2004).
Biofilm present on dentures can be controlled by mechanical methods, chemical and mechanical-chemical sanitization. Several methods are available for disinfection of dental prostheses. Chemical solutions, such as glutaraldehyde, sodium hypochlorite, chlorine dioxide, iodine, alcohol and chlorhexidine are frequently used for disinfection purposes; however, there are several drawbacks in the use of such chemical agents for disinfection of prosthesis.
Despite possessing bactericidal action, these different chemical disinfectants have different effects on the surface characteristics and color of denture resins (Ma et al., 1997). A classic study has shown that the association between brushing with toothpaste and commercial cleanser soaking is a popular method of cleaning dentures (Jagger & Harrison, 1995). However, the use of cleaning tablets for acrylic denture overnight storage reduces denture biofilm mass and pathogenicity when compared to dry and water preservation, and may contribute to the overall systemic health (Duyck et al., 2013).
The procedures used in clinical therapy for denture stomatitis comprises mechanical removal of biofilm from the surface of the denture base and underlying mucosa associated to antiseptics and disinfectants. Furthermore, it is also recommended the use of systemic antifungal drugs and denture disinfection by microwave energy (Salerno et al., 2011). Although the microwave irradiation is still restricted, microwave irradiation has been suggested in the literature by classic studies (Rohrer & Bulard, 1985; Baysan et al., 1998; Webb et al., 1998 and Dixon et al., 1999) and more recent works (Brondani et al., 2012; Silva et al., 2012 and Silva et al., 2013) as a promising method for sterilizing acrylic resin denture bases contaminated with Candida albicans.
Based on these considerations, the purpose of this study was to verify the effect of the microwave disinfection on different types of denture base acrylic resins (conventional, microwaved and boiled) in presence of Candida biofilms (C. albicans, C. dubliniensis and C. tropicalis). The work hypothesis was that microwave energy should cause similar effects in the biofilms formed on different acrylic resins for denture bases.
Materials and Methods
Preparation and Sterilization of Samples:
Circular wax patterns (40 mm in diameter and 4 mm in high) were conventionally included in plastic (Classico Dental Products, Sao Paulo, SP, Brazil) or metallic (Safrany Metallurgy, Sao Paulo, SP, Brazil) flasks with type III dental stone (Herondent; Vigodent, Petropolis, RJ, Brazil). In the dental stone molds left by the wax patterns were conventionally made 30 samples for each type of acrylic resin (n= 90). Classico (Classico Dental Products), Onda-Cryl (Classico Dental Products) and QC-20 (Dentsply, Petropolis, RJ, Brazil) heat-curing acrylic resins were proportioned, manipulated and polymerized according to the manufacturers’ instructions.
The acrylic resin samples were sterilized with ethylene oxide (ACECIL Sterilization Center, Campinas, SP, Brazil). Afterwards, the samples were manipulated up to 15 days after sterilization because beyond this period the sterilization would be compromised due to expiration date.
Microorganisms and Growth Conditions:
Three species of Candida were tested for a more understandable study in relation to the disinfection of microorganisms responsible for oral candidiasis: C. albicans ATCC 90028, C. dubliniensis ATCC 7987 and C. tropicalis ATCC 4563.Candida species are the most common pathogens form of oral infection for the development of denture stomatitis (Dorko et al., 2001; Dar-Odeh & Shehabi, 2003; Web et al., 2005; Ganguly & Mitchell, 2011 and Salerno et al., 2011). The microorganisms were originally obtained from the Microbiology and Immunology Laboratory, Department of Oral Diagnosis, Piracicaba Dental School, UNICAMP. Candida species were cultivated on Sabouraud Dextrose Agar (Difco Laboratories, Detroit, MI, USA) at 37ºC for 24 hours in aerobic condition. Microbial suspensions were obtained from single colonies isolated on agar plates, inoculated in the appropriate broth for overnight cultures at 37ºC. Candida spp. was grown in Sabouraud Dextrose Broth (SDB). Cells of the resultant cultures were harvested, washed twice with phosphate-buffered saline (PBS, pH 7.2), centrifuged at 5000x g for 5 minutes and re-suspended in phosphate-buffered saline (PBS, pH 7.2). Microbial suspensions were spectrophotometrically (BioPhotometer plus, Eppendorf, Hauppauge, NY, USA) standardized to a concentration of 1×108 cells/mL.
Assays Biofilm Formation in Samples:
Pre-sterilized polystyrene flat-bottomed microtiter plates were used for Candida species biofilm production. Two mL of standard cell suspensions (108 cells/mL) were added to each well containing the sterile resin specimen. The plate was incubated at 37ºC for 90 minutes to promote microorganism adherence on the resin specimen. Afterwards, the specimens were transferred to other wells and the non-adherent cells were removed by washing with 2 mL PBS. Two mL of sabouraud dextrose fresh broth was added to each well to promote biofilm growth. After incubation at 37ºC for 48 hours under aerobic conditions, the plates were removed from the incubator and the wells washed with PBS. Afterwards, the specimens were transferred to a tube containing PBS and sonicated for 20 minutes to disrupt the cells aggregate of the biofilm. The suspension containing the detached biofilm cells was vortexed, diluted and plated on sterile Petri dishes containing selective media for Candida spp. at 37ºC for 48 hours under aerobic conditions.
Samples of resin were transferred to a tube containing 1 ml PBS and then sonicated to disrupt the cell pellet biofilm. The suspension containing cells isolated from the biofilm were vortexed and 100 µl of this suspension was plated and dilutions (10x, 100x, 1000x) were also performed and seeded in sterile Petri plates containing Sabouraud Dextrose Agar medium (SDA) (Silva et al., 2010 and Marra et al., 2012). Count colonies on each Petri dish were quantified using a CP 600 more digital colony counter (Phoenix, Araraquara, SP, Brazil). The results were given as colony forming unit per milliliter (CFU/mL).
Microwave Irradiation:
For each type of acrylic resin, three groups (n=30) were obtained: 1) samples inoculated with C. albicans; 2) samples inoculated with C. dubliniensis and 3) samples inoculated with C. tropicalis. After biofilm growth, the samples of each group were submitted to microwave irradiation, and this procedure was carried out for the following subgroups (n=10): I — resin samples irradiated in triplicate, II — Non-irradiated resin samples (positive control) and III — resin samples without contamination (negative control).
Subsequent to the incubation period, the samples were removed from the wells of plastic plates containing culture media, washed in PBS and immersed individually in sterile beaker containing 10 mL of sterile water. Each beaker was microwave irradiated (Continental; Amazonas, MA, Brazil) at 650 W for 3 minutes (Sanitá et al., 2008). After irradiation, each sample was aseptically removed from the beaker, washed, transferred to a tube containing PBS, and sonicated for 20 minutes to disrupt the biofilm cell aggregates. The solution with detached cells of the biofilm was vortexed and diluted, and plated on sterile Petri dishes containing medium for Candida spp. at 37ºC for 48 hours under aerobic. The media used for plating for Candida spp. were Sabouraud dextrose agar with 5 µg/mL of chloranphenicol. After incubation, colony counts of each Petri dish were quantified using a digital colony counter (CP 600 Plus).
Statistical Analysis:
Kruskal-Wallis test was used for evaluated the mid-points of the microorganism accounts in each one of the experimental and control groups. The data were submitted to nonparametric multiple comparison of paired means for Student-Newman-Keuls test. A significance level of 5% was used to analyze the difference among average positions.
Results
The non-irradiated samples (control) revealed microbial growth in all resins after incubation for 48 hours. In each treatment, the comparison between Candida species in same resin was not significant, as well as between resins in the same Candida specie. The disinfected samples showed no viable colonies in all Candida species assessed after incubation, for all acrylic resins. In each treatment, the comparison between Candida species in same resin was not significant, as well as between resins in the same Candida specie. When non-irradiated (control) and disinfection treatments were compared, microwaved specimens showed decrease in the number of viable colonies formed by C. tropicalis on all resin types, C. dubliniensis on the Classico and QC-20 resins, and C. albicans on the Onda-Cryl and QC-20 resins (Table 1).
Table 1 – Means (SD) of CFU/mL in Acrylic Resins Contaminated with Candida Species for Control and Microwaved Treatments
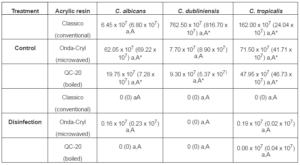
Means followed by distinct letters are statistically different (5%). Lowercase letters in each row compare Candida species in each treatment for same resin (Kruskal-Wallis). Uppercase letters in each column compare different resins in the same Candida species and treatment (Kruskal-Wallis). (*) Asterisks compare treatments in the same Candida species and resins. (Kruskal-Wallis/Student-Newman-Keuls).
Discussion
The current study evaluated the effect of microwave disinfection in biofilms of Candida species (C. albicans, C. dubliniensis and C. tropicalis) formed in acrylic resin (Classico, Onda-Cryl and QC-20). It was demonstrated that the non-irradiated samples presented microbial growth after incubation at 37°C for 48 hours. Microwaved samples at 650 W for 3 minutes showed different decreases in the number of viable colonies for biofilms formed by C. albicans, C. dubliniensisand C. tropicalis (Table 1). Based on these results, the work hypothesis that microwave energy should cause similar effects in all biofilms formed on different acrylic resins was partially confirmed.
The findings of the present investigation are in agreement with classic studies in which was verified that microwave irradiation is effective for cleaning and disinfecting dental instruments (Rohrer & Bulard, 1985), resilient relines and denture bases (Baysan et al., 1998; Dixon et al., 1999), and oral prosthesis (Webb et al., 1998). More recent studies also verified that microwave energy was efficient for denture disinfection (Brondani et al., 2012), denture disinfection associated to antifungal therapy (Silva et al., 2012) and as an alternative method for disinfection of different types of denture base acrylic resins (Silva et al., 2013). Based on these aforementioned studies, the microwave disinfection of dentures infected with these microorganisms appears to contribute significantly for prevention of oral stomatitis and cross-contamination.
There was no previous sterilization of the samples in this current investigation because the aim was to verify the effect of the microwave disinfection on acrylic resins contaminated by Candida biofilms and not affected by planktonic cells of these microorganisms. It has been noted in previous study that the symptoms of denture stomatitis often return shortly after treatment, suggesting that C. albicans found in infestations is more resistant to antifungal drugs action (Chandra et al., 2001). This allegation reinforces the assumption that microwave energy should be used aiming the disinfection of acrylic resin denture base contaminated by Candida species.
An in vitro study showed microwave sterilization for all dentures contaminated by different species of Candida (C. albicans, C. glabrata, C. tropicalis, C. krusei and C. dublinensis) isolated from standard cultures or from HIV positive pattern (Sanitá et al., 2008). In general, these findings are in accordance with the results of the present study showing microwave disinfection of acrylic resins contaminated by biofilms of C. albicans, C. dublinensis and C. tropicalis. In addition, results from an in vitro investigation showed that microwave irradiation was also effective in disinfection of dental prosthesis contaminated with S. aureus and P. aeruginosa species (Dovigo et al., 2009).
Study reported in the literature had recommend that the microwave disinfection should be with the samples immersed in water (Dixon et al., 1999), indicating that the action on the pathogens can be purely thermal. Scanning electron microscopy analysis showed that microwave irradiation induced a morphological modification in the Candida cells. Therefore, the longer the exposure time the greater alterations and the microorganism fracture occurs after 9 minutes (Rosaspina et al., 1994). The candidal adhesion to denture base acrylic resins differs depending on the quality of the material used as well as the Candida species in question. Thus, it was also observed that, in general, fewer cells adhere to the heat-polymerized resin when compared to auto- polymerized resin (He et al., 2006).
In the present study, statistically significant differences were showed between treatments for the number of viable colonies formed by C. tropicalis on all resins, C. dubliniensis on the Classico and QC-20 resins, and C. albicans on the Onda-Cryl and QC-20 resins (Table 1). This result is significant considering that re-infestation of the denture surface and infection of the adjacent soft tissue were delayed dramatically in patients whose dentures were microwaved compared with those whose dentures were chemically disinfected (Banding & Hill, 2001).
All acrylic resins studied are thermo-polymerized; however, they have different temperature and time of polymerization. Previous study has shown that this condition can cause different effects on the cell adhesion levels and the candidal adherence to denture base differs depending on the quality of the acrylic resin used, as well as the Candida species in question (He et al., 2006).
Dental prostheses disinfection methods are intended to eliminate pathogenic microorganisms no causing adverse effects on physical and mechanical properties of acrylic resins. Exposure to microwaves for 5 minutes of samples immersed in water did not result in a significant change in hardness for denture resin-based materials (Dixon et al., 1999), and simulated microwave disinfection at 650 W for 3 minutes did not cause misfit of acrylic resin denture bases immersed in water (Consani et al., 2007). Based on these considerations, it is possible to assume that the microwave disinfection method used in this study should not cause deleterious effects on the mechanical properties of resins for denture base. However, further studies should be conducted to analyze the effect of repeated microwave disinfection in Candida biofilms infesting different types of acrylic resins, and the possible changes in their mechanical properties.
Reinforcing this assumption, it was showed accumulative effects due to two protocols of disinfection by microwave energy on surface roughness and adaptation of complete denture bases made with microwaved and boiled resins. The results showed that microwave irradiation at 690 W for 6 minutes promoted changes on surface roughness and adaptation of the denture made with both resin types, but the clinical use would not be affected by the magnitude of the changes (Campos et al., 2009).
Inoculed by different Candida species (C. albicans, C. dubliniensis and C. tropicalis), previous study has shown that the microwave irradiation at 650 W for 3 minutes was effective for disinfection of different acrylic resin types from a same manufacturer (Silva et al., 2013). In the same methodological conditions, the current study showed that the microwave effectivity was also evident for biofilms from Candida species (C. albicans, C. dubliniensis and C. tropicalis) inoculated on acrylic resins from different manufactures. Thus, it is possible to suggest that the present disinfection protocol would be appropriate in promoting the inactivation of microorganisms found in denture bases. Previous study shows that the cumulative effects of different microwave power levels did not cause significant changes for many mechanical properties of denture base resins (Senna et al., 2011).
The inactivation of biofilms of different yeasts using microwave oven is directly related to microorganism species and material to be sterilized as well as exposure time and irradiance, making it difficult to standardize the procedures used in other studies. Most studies report microwave sterilization of strains of Candida differing from the present study, which investigated the disinfection of various Candida species biofilms. Thus, different energy, frequencies and periods of irradiation must be assessed in the future to identify ideal relation time-energy for microwave disinfection of biofilms formed by Candida species.
Clinical assessments of the effects of microwave disinfection on the retention and stability of denture base are also required. Investigations should be made about the long term effects of disinfection techniques on the hardness and roughness of acrylic resins used in complete and partial removable dentures. Although there is need for further confirmatory investigations, the results of this study suggest that denture microwave disinfection seems to be a promising method on the prevention of Candida infestation.
Conclusion
Microwaved samples at 650 W for 3 minutes showed different decreases in the number of viable colonies for biofilms formed by C. albicans, C. dubliniensis and C. tropicalis. In general, microwave irradiation at 650 W for 3 minutes can be an alternative method to disinfect different denture acrylic resins contaminated by different Candida species.
References
Banting, D. W. & Hill, S. A. (2001). “Microwave Disinfection of Dentures for the Treatment of Oral Candidiasis,” Special Care in Dentistry, 21 (1) 4-8.
Publisher – Google Scholar
Baysan, A., Whiley, R. & Wright, P. S. (1998). “Use of Microwave Energy to Disinfect a Long-Term Soft Lining Material Contaminated with Candida Albicans or Staphylococcus Aureus,” Journal of Prosthetic Dentistry, 79 (4) 454-458.
Publisher – Google Scholar
Brondani, M. A., Samim, F. & Feng, H. (2012). “A Conventional Microwave Oven for Denture Cleaning: A Critical Review,”Gerodontology, 29 (2) e6-e15.
Publisher – Google Scholar
Budtz-Jögersen, E. (1990). “Etiology, Pathogenesis, Therapy, and Prophylaxis of Oral Yeast Infections,” Acta Odontologica Scandinavica, 48 (1) 61-69.
Publisher – Google Scholar
Campos, M. A. P., Kochenborger, C., Silva, D. F. F., Teixeira, E. R. & Shinkai, R. S. A. (2009). “Effect of Repeated Microwave Disinfections on Surface Roughness and Baseplate Adaptation of Denture Resins Polymerized by Different Techniques,” Journal of Dental Sciences, 24 (1) 40-44.
Publisher
Chandra, J., Mukherjee, P. K., Leidich, S. D., Faddoul, F. F., Hoyer, L. L., Douglas, L. J. & Ghannoum, M. A. (2001). “Antifungal Resistance of Candidal Biofilms Formed on Denture Acrylic in Vitro,” Journal of Dental Research, 80 (3) 903-908.
Publisher – Google Scholar
Coelho, C. M. P., Sousa, Y. T. C. S. & Dare, A. M. Z. (2004). “Denture-Related Oral Mucosal Lesions in a Brazilian School of Dentistry,” Journal of Oral Rehabilitation, 31 (2) 135-139.
Publisher – Google Scholar
Consani, R. L. X., Mesquita, M. F., Nobilo, M. A. A. & Henriques, G. E. P. (2007). “Influence of Simulated Microwave Disinfection on Complete Denture Base Adaptation Using Different Flask Closure Methods,” Journal of Prosthetic Dentistry, 97 (3) 173-178.
Publisher – Google Scholar
Dar-Odeh, N. S. & Shehabi, A. A. (2003). “Oral Candidosis in Patients with Removable Dentures,” Mycoses, 46 (5-6) 187-191.
Publisher – Google Scholar
Dixon, D. L., Breeding, L. C. & Faler, T. A. (1999). “Microwave Disinfection of Denture Base Materials Colonized with Candida Albicans,” Journal of Prosthetic Dentistry, 81 (2) 207-214.
Publisher – Google Scholar
Dorko, E., Jenca, A., Pilipcinec, E., Danko, J., Svicky, E. & Tkacikova, L. (2001). “Candida-Associated Denture Stomatitis,” Folia Microbiologica, 46 (5) 443-446.
Publisher – Google Scholar
Dovigo, L. N., Pavarina, A. C., Ribeiro, D. G., Oliveira, J. A., Vergani, C. E. & Machado, A. L. (2009). “Microwave Disinfection of Complete Dentures Contaminated in Vitro with Selected Bacteria,” Journal of Prosthodontics, 18 (7) 611-617.
Publisher – Google Scholar
Duyck, J., Vandamme, K., Muller, P. & Teughels, W. (2013). “Overnight Storage of Removable Dentures in Alkaline Peroxide-Based Tablets Affects Biofilm Mass and Composition,” Journal of Dentistry. Doi: 10.1016/j.jdent.2013.08.002. [Epub ahead of print].
Publisher – Google Scholar
Ganguly, S. & Mitchell, A. P. (2011). “Mucosal Biofilms of Candida Albicans,” Current Opinion in Microbiology, 14 (4) 380-385.
Publisher – Google Scholar
Gendrau, L. & Loewy, Z. G. (2011). “Epidemiology and Etiology of Denture Stomatitis,” Journal of Prosthodontics, 20 (4) 251-260.
Publisher – Google Scholar
He, X. Y., Meurman, J. H., Kari, K., Rautemaa, R. & Samaranayake, L. P. (2006). “In Vitro Adhesion of Candida Species to Denture Base Materials,” Mycoses, 49 (2) 80-84.
Publisher – Google Scholar
Jagger, D. C. & Harrison, A. (1995). “Denture Cleaning — The Best Approach,” British Dental Journal, 178 (11) 413-417.
Publisher – Google Scholar
Mandali, G., Sener, I. D., Turker, S. B. & Ulgen, H. (2011). “Factors Affecting the Distribution and Prevalence of Oral Mucosal Lesions in Complete Denture Wearers,” Gerodontology, 28 (2) 97-103.
Publisher – Google Scholar
Marra, J., Paleari, A. G., Rodriguez, L. S., Leite, A. R. P., Pero, A. C. & Compagnoni, M. A. (2012). “Effect of an Acrylic Resin Combined with an Antimicrobial Polymer on Biofilm Formation,” Journal of Applied Oral Science, 20 (6) 643-648.
Publisher – Google Scholar
Ma, T., Johnson, G. H. & Gordon, G. E. (1997). “Effects of Chemical Disinfectants on the Surface Characteristics and Color of Denture Resins,” Journal of Prosthetic Dentistry, 77 (2) 197-204.
Publisher – Google Scholar
Pires, F. R., Santos, E. B. D., Bonan, P. R. F., Almeida, O. P. & Lopes, M. A. (2002). “Denture Stomatitis and Salivary Candida in Brazilian Edentulous Patients,” Journal of Oral Rehabilitation, 29 (11) 115-119.
Publisher – Google Scholar
Pusateri, C. R., Monaco, E. A. & Edgerton, M. (2009). “Sensitivity of Candida Albicans Biofilm Cells Grown on Denture Acrylic to Antifungal Proteins and Chlorhexidine,” Archives of Oral Biology, 54 (6) 588-594.
Publisher – Google Scholar
Ramage, G. & Lopez-Ribot, J. L. (2005). “Techniques for Antifungal Susceptibility Testing of Candida Albicans Biofilms,”Methods in Molecular Medicine, 118 71-79.
Publisher – Google Scholar
Rohrer, M. D. & Bulard, R. A. (1985). “Microwave Sterilization,” The Journal of the American Dental Association, 110 (1) 194-198.
Publisher – Google Scholar
Rosaspina, S., Salvatorelli, G., Anzanel, D. & Bovolenta, R. (1994). “Effect of Microwave Radiation on Candida Albicans,”Microbios, 78 (3) 55-59.
Publisher – Google Scholar
Salerno, C., Pascale, M., Contaldo, M., Esposito, V., Busciolano, M., Milillo, L., Guida, A., Petruzzi, M. & Serpico, R. (2011). “Candida-Associated Denture Stomatitis,” Medicina Oral Patologia Oral y Cirugia Bucal, 16 (2) e139-143.
Publisher – Google Scholar
Sanitá, P. V., Vergani, C. E., Giampaolo, E. T., Pavarina, A. C. & Machado, A. L. (2008). “Growth of Candida Species on Complete Dentures: Effect of Microwave Disinfection,” Mycoses, 52 (2) 154-160.
Publisher – Google Scholar
Senna, P. M., Silva, W. J., Faot, F. & Del Bel Cury, A. A. (2011). “Microwave Disinfection: Cumulative Effect of Different Power Levels on Physical Properties of Denture Base Resins,” Journal of Prosthodontics, 20 (8) 606-612.
Publisher – Google Scholar
Silva, M., Consani, R., Sardi, J., Mesquita, M., Macedo, A. & Takahashi, J. (2013). “Microwave Irradiation as an Alternative Method for Disinfection of Denture Base Acrylic Resins,” Minerva Stomatologica, 62 (1-2) 23-29.
Publisher
Silva, M. M., Mima, E. G., Colombo, A. L., Sanita, P. V., Jorge, J. H., Massucato, E. M. & Vergani, C. E. (2012). “Comparison of Denture Microwave Disinfection and Conventional Antifungal Therapy in the Treatment of Denture Stomatitis: A Randomized Clinical Study,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 114 (4) 469-479.
Publisher – Google Scholar
Silva, W. J., Seneviratne, J., Samaranayake, L. P. & Del Bel Cury, A. A. (2010). “Bioactivity and Architecture of Candida Albicans Biofilms Developed on Poly(Methyl Methacrylate) Resin Surface,” Journal of Biomedical Materials Research Part B: Applied Biomaterials, 94 (1) 149-156.
Publisher – Google Scholar
Torres, S. R., Peixoto, C. B., Caldas, D. M., Silva, E. B., Akiti, T., Nucci, M. & Uzeda, M. (2002). “Relationship between Salivary Flow Rates and Candida Counts in Subjects with Xerostomia,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 93 (2) 149-154.
Publisher – Google Scholar
Webb, B. C., Thomas, C. J., Harty, D. W. S. & Willcox, M. D. P. (1998). “Effectiveness of Two Methods of Denture Sterilization,” Journal of Oral Rehabilitation, 25 (6) 416-423.
Publisher – Google Scholar
Webb, B. C., Thomas, C. J. & Whittle, T. (2005). “A 2-Year Study of Candida-Associated denture Stomatitis Treatment in Aged Care Subjects,” Gerodontology, 22 (3) 168-176.
Publisher – Google Scholar
Wilson, J. (1998). “The Aetiology, Diagnosis and Management of Denture Stomatitis,” British Dental Journal, 185 (8) 380-384.
Publisher – Google Scholar



