Introduction
Canola, palm, soybeans, and sunflower seeds contribute up to 79% of the global vegetable oil on the market (Dyer et al. 2008). Due to both the increasing demand for food consumption in conjunction with the increased interest in bio-fuel feedstock’s, consumption of vegetable oil has increased 50% over the past decade (Lu et al. 2011). Increased market demand coupled with consequent rise in oil prices are forcing industries that rely on natural fats and oils for bio-based formulations i.e. bio-fuel, surfactants, and lubricants, to seek new feedstock’s for products and applications. One oil source gaining interest is canola (Meier et al. 2007). The potential of canola as a bio-oil source has been well demonstrated (Sensoz, Angın, Yorgun 2000; Yu et al. 2010; Suqi et al. 2014).
Our goal was to ease the transition of canola production into the southeastern US. To accomplish this we need to find the highest yielding varieties suitable to our climate, as well as determine optimum fertilizer application rate. We evaluated two winter canola varieties to determine their yield potential. Producers not only rely on high yielding varieties, but also on nutrient management practice systems to supply necessary nutrition to crops. Efficient nutrient management practices include sufficient and timely introduction of needed nutrients, as well as minimal reliance on excess nutrient supplementation. Previous research has shown that when nitrogen (N) fertilizer application to canola is increased, a noticeable reduction in oil content is observed, while at the same time seed yield, energy production, CO2 storage, and crude protein content are all increased (Rathke, Christen & Diepenbrock 2005). Therefore to maintain a profitable oilseed crop, producers need to manage optimal nitrogen application to produce high oil content. We investigated the effect of 3 different (N-P-K) applications and their differential effect on yield response. In conjunction with the rate of application, we investigated foliar Soysoap™ application during a critical growth time (flowering) for canola. Soysoap is a bio-based surfactant currently available on the market for multiple agricultural applications that has the potential for reducing nitrogen supplementation while increasing efficiency of nutrient uptake. Our goal was to achieve increased seed and oil yields while simultaneously reducing fertilizer inputs.
Canola oil quality is an important aspect that needs to be addressed in order to determine the optimum nutrient management practice to achieve the highest value. For this reason, this study examined not only the growth profile resulting from the growth system but the production value of the canola oil. The most important quality parameter for lipid production in oilseed crops is determined by its fatty acid composition. Oil quality is determined by its oleic, linoleic, and erucic acid contents (Aslam et al. 2009; Schierholt & Becker 2011; Rad & Zandi 2012). The concentration of these fatty acids is affected by genomic differences between varieties and environmental influences. Environmental conditions (heat and salinity) as well as management practices (planting date and fertilizer application) affect fatty acid metabolism. Fatty acid profiles are susceptible to seasonal variation due to climatic influences such as temperature. Temperature fluctuations during crop production can delay seed maturation altering fatty acid profiles (Deng & Scarth 1998). Canola is most vulnerable to fatty acid profile changes during flowering (2-5 week duration). During this period, the seed’s cotyledons experience changes in fatty acid composition, accompanied by rapid oil accumulation (Aksouh-Harradj, Campbell & Mailer 2006). Nutrient supply also has a significant influence on biochemical reactions that affect fatty acid development (DeBonte et al. 2012). Researchers have noted that increasing N fertilization from 100 kg/ha to 150 kg/ha increased palmitic, stearic, oleic, linoleic, linolenic, arachidic, eicosenoic, behenic, erucic and nervonic fatty acid concentrations (Zheljazkov et al. 2012).
Objectives
Primary: Determining the effectiveness of cultivar selection, and fertilizer treatment application on yield response.
Secondary: Investigating fatty acid profiles of individual treatments partitioned by the effects of cultivar selection, and fertilizer treatment application.
Methodology
Experimental Design
The experiment was conducted at North Carolina Agricultural and Technical State University Research Farm which is located 223m above the sea level. The current climate hardiness zone is classified as zone 7A. The experiment was conducted on a soil type classified as a Mecklenburg Sandy Clay Loam (Taxonomic class: Fine, mixed, active, Thermic Ultic Hapludalfs). The soil is well drained with 6-10% slopes, and moderately eroded. The experimental design was a 2 X 4 split plot randomized complete block, with 2 cultivars (Virginia and DKW 46-15) as main plots, and fertilizer rates (4) as sub plot, in 4 replications. Fertilizer rates included: (T4) 140-56-168 (N-P-K) kg/ha (100% dose fertilizer (DF)+Soysoap), (T3) 140-56-168 (N-P-K) kg/ha (100% DF), (T2) 70-28-84 kg/ha (N-P-K) + Soysoap (50% DF+Soysoap) and (T1) 0-0-0 kg/ha (N-P-K) (control). Each subplot was 6 m x 10 m. Canola was planted on October 1st (2010) and October 7th (2011). Canola was manually harvested on May 21st (2011), and May 31st (2012).
Oil extraction
Solvent extraction was performed using a Soxhlet apparatus using a Pyrex® Allihn condenser with a 500ml round bottom flask heated with analytical grade hexane. Lipid extraction was completed following a modification of AOAC Official Method;948.22 (Venkatachalam 2006). Forty grams of the seed was pulverized using a coffee grinder for 15 seconds. Seed cake was then placed into a cellulose extraction thimble (43mm x 123mm) and extracted with 400ml of hexane which was poured into the still pot of the soxhlet apparatus. The temperature was brought to 69ºC (boiling point of hexane) and the extraction tube was inserted along with the condenser tube. The thimble was then placed into the condenser tube. Reflux took place for 6 hours. The still pot was removed and the contents poured into an uncapped 500ml beaker and the hexane solvent was allowed to evaporate by placing the beaker in a fume hood at ambient temperature. Residual oil samples were then weighed and lipid content determined by AOAC 920.39. Number of extractions were based on the number of plots that were separated by both fertilizer treatment and variety (N=32). Each plot was extracted in triplicate. Oil percentage was calculated by placing the oil extracted during chemo-solvent extraction into an uncapped 500ml beaker which was then placed in a Fischer scientific isotemp oven for 24 hours at 80oC. After drying was complete, oil weight was determined.
Fatty Acid Analysis
Fatty acid analyses were performed using oil samples collected from the extraction process using procedures detailed by Kaluzny et al. (1985). Samples from oil extraction were weighed (between 0.23-0.27g) and then placed into a 15ml vial. 10 ml of a 2:1 chloroform: methanol mixture was added to each sample and then vortexed for 30 seconds and allowed to settle for 5 minutes. The samples were then centrifuged at 15oC for 10 minutes at 3,000 rpm. A 3 ml portion from the bottom layer of the resulting sample mixture from these samples was extracted and applied to a pre-conditioned supelco supelclean LC-NH2 SPE cartridge. The samples were eluted with 6ml hexane followed by 3ml of a 2:1 chloroform: methanol mixture and then treated with 3ml of a 2:1 chloroform/isopropanol mixture to the cartridge to remove the neutral lipids. All fractions containing pure solvent were discarded. The free fatty acids were then eluted off of the LC-NH2 column using 6 ml of 2% acetic acid in diethyl ether and collected in a 10ml sample vial. These prepared samples were then analyzed on Gas Chromatography Thermo Fisher Trace GC equipped with Triplus Autosampler using nukol megabore column.
Statistical Software and Analysis
Data collected from each plot (N=32) on seed and oil solvent yield were analysed using a PROC GLM procedure of SAS (version 9.2; SAS Institute Inc., Cary, NC) using a split plot design. Oil quality was accessed through total fatty acid analysis that evaluated 5 important fatty acid concentrations related to canola production (Oleic, Linoleic, Linolenic, Palmitic, and Erucic). All analyses conducted used Duncan’s multiple range test for post hoc analysis (α=0.05).
Results
Canola yield (seed and oil) responses are dominated by two primary factors; genomics and nutrient management practices. Our study concluded that seed and solvent extracted oil yields (kg/ha) were significantly influenced by cultivar selection and fertilizer treatment application (Table 1).
Table 1: P-Values observed during individual split-plot analyses
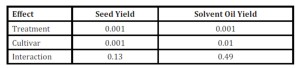
” =Each column represents individual analyses of seed and oil yield response due to fertilizer treatment applied and cultivar selected. Significance was determined at (α=0.05%).
Cultivar selection was a pivotal factor affecting yield response for both observed seed and oil yields. DKW 46-15 statistically produced superior yields compared to the Virginia cultivar (Table 2). Fertilizer treatment was also a significant influence which affected yield response (Table 1).
Table 2: Cultivar Effect on Seed and Oil Yields (kg/ha)
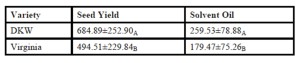
” =Each column represents individual analyses of cultivar effect on seed and oil yield response. Mean followed by different letters in each column are significantly different (Duncan multiple range test at α=0.05%).
The highest Duncan grouping observed in both seed and oil yield response was obtained with the application of treatment T4 (Table 3). An interesting trend was observed between similar yield
responses between treatments T2, and T3 (Table 3). Treatment T2 was applied with half the fertilizer (N-P-K) as treatment T3 however, T2 received soysoap application (Table 3).
Table 3: Fertilizer Treatment Effect on Seed and Oil Yields (kg/ha)
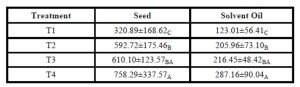
” =Each column represents individual analyses of fertilizer treatment effect on seed and oil yield response. Mean followed by different letters in each column are significantly different (Duncan multiple range test at α=0.05%).
Both seed and oil yield shared a similar response in which the control or nil-applied fertilizer treatment produced significantly lower yields compared to the other treatments evaluated.
Fatty Acid Profile
After yield response was determined, we evaluated quality characteristics of solvent extracted oil. The effect of fertilizer treatment and cultivar selected on fatty acid profile was the focus of the analyses (Table 4). Genetic influences were shown to be the strongest influence on fatty acid profiles between the two varieties (Table 4). The Virginia cultivar displayed significantly higher erucic fatty acid concentrations compared to DKW 46-15 (Table 5). We found that the rate of fertilizer treatment applied had little effect on fatty acid profiles between the two canola cultivars studied (Table 4).
Table 4: P-Values observed during individual split-plot analyses
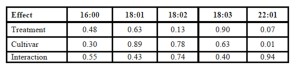
” = (16:0) Palmitic FA, (18:1) Oleic FA, (18:2) Linoleic FA, (18:3) Linolenic FA, (22:1) Erucic FA. Significance was determined at (α=0.05%).
Table 5: Cultivar effect on fatty acid analyses
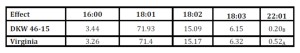
” = Mean followed by different letters in each column are significantly different (Duncan multiple range test at α=0.05%), (16:0) Palmitic FA, (18:1) Oleic FA, (18:2) Linoleic FA, (18:3) Linolenic FA, (22:1) Erucic FA.
Discussion
Yield Response
Bio-fuel refineries are in an early developmental stage in North Carolina. For the stabilization of market demand to occur, a reliable feedstock needs to be made readily available. Therefore, we established a cultivar and nutrient management field trail to enhance canola production in the south eastern US. Seed and oil yield are the two most important aspects of canola production. Seed quantity produced is directly affected by genomic influences such as cultivar selection and nutrient management practices (rate of fertilizer applied). Our study evaluated two different winter canola cultivars; Virginia and DKW 46-15. We found that DKW 46-15 produced higher seed and oil yields compared to the Virginia variety.
Another consideration affecting crop productivity and operation cost is nutrient application. We evaluated 3 different fertilizer rates to see the response from (N-P-K) application, as well as the effect of soysoap application on yield. Surfactants such as Soysoap may potentially affect absorption/desorption processes which affect nutrient and water absorption mechanisms. Treatments T3 -without soysoap and T4 —with soysoap produced similar yields between the cultivars tested. However, the most dramatic response observed occurred when plots were applied with treatment T4 in comparison to T2, or T1. Underlying mechanisms such as increased water and nutrient delivery may be the cause of increased yield response associated with soysoap application. However, to further confirm this on a physiological level goes beyond the scope of the current field trial evaluation.
Biofuel Feedstock Quality
Producers have several factors which influence their options for oil extraction. Solvent extraction is more efficient in terms of quantity of oil produced compared to mechanical pressing. However, negative drawbacks using solvent extraction consist of longer and more expensive processing, production of hazardous waste, and alteration of end quality output (Buenrostro & López-Munguia 1986; Wei et al. 2012). Fatty acid composition impacts several characteristics of biodiesel quality such as: flashpoint, viscosity, cetane number, cloud point, pour point, calorific value, acid value, ash content, and cold flow properties. Two primary areas of interest for bio-fuel feedstock production involve combustion and storage characteristics. We evaluated fatty acid profiles by cultivar selection and fertilizer treatment effects.
Over time plant breeders developed Brassica napus to contain lower amounts of erucic acid due to detrimental effects when used as food or fodder; however, erucic fatty acid has important industrial applications, therefore evaluating cultivar performance can help producers select appropriate end use varieties (Nosenko, Hutsalo et al. 2013). Erucic fatty concentrations were consistently higher from oil originating from the Virginia cultivar compared to DKW 46-15. Oil containing increased saturation of erucic fatty acids will require additional processing for biodiesel production which will increase production cost. Having higher erucic acid concentrations could be adventitious depending on the oil end use, high fatty acid concentrations may be favourable; oleic and erucic fatty acids have been previously sought for the utilization in bio-based polyesters (Yang et al. 2009).
Conclusion
Producing high value fatty acids through oilseed crops can help supplement and replace the use of non-sustainable petroleum and oceanic resources (Cahoon et al. 2007). As we explore oil seed feedstocks for bio-fuel production as well as other manufacturing purposes determining fatty acid composition, management practices, and/or choice of cultivars to plant that optimize canola production will help producers and suppliers better meet market demands. We observed greater yields (seed and oil) with DKW 46-15 compared to the Virginia cultivar. Fertilizer treatment trends were noted in yield response with soysoap application; treatments T2 -with soysoap (reduced fertilizer rate), and T3 -without soysoap (increased fertilizer rate) produced similar yields, and a higher yield response was observed for treatment T4 compared to T2, and T1. The exact role of soysoap in improving the yield could not be determined within the scope of this study. Future studies should be directed at smaller (N-P-K) application rates in conjunction with soysoap application. Oil quality was primarily affected by genomic influences due to cultivar selection. Erucic fatty acid concentrations were statistically higher regardless of the extraction method used.
Acknowledgment
Funding was provided by Evans Allen. The review of the manuscript by Dr. J. Emrani and Dr. K. Gruber is appreciated. This work could not have been completed without the assistance of John Carver and Bryce Holmes.
References
Aksouh-Harradj, N. M., Campbell, L. C., and Mailer, R. J. (2006). “Canola response to high and moderately high temperature stresses during seed maturation.” Canadian Journal of Plant Science, 86(4), 967-980.
Publisher – GoogleScholar
2. Aslam, M. N., Nelson, M. N., Kailis, S. G., Bayliss, K. L., Speijers, J., and Cowling, W. A. (2009). “Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed mediterranean-type environments.” Plant Breeding, 128 (4) 348-355
Publisher – GoogleScholar
3. Buenrostro, M. and A. López-Munguia (1986). “Enzymatic extraction of avocado oil.” Biotechnology letters . 8 (7) 505-506.
Publisher – GoogleScholar
5. Cahoon, E. B., Shockey, J. M., Dietrich, C. R., Gidda, S. K., Mullen, R. T., and Dyer, J. M. (2007). “Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux.” Current opinion in plant biology, 10 (3) 236-244.
Publisher – GoogleScholar
6. DeBonte, L., Iassonova, D., Liu, L., and Loh, W. (2012). “Commercialization of high oleic canola oils.” Lipid Technology, 24 (8) 175-177.
Publisher – GoogleScholar
7. Deng, X., and Scarth, R. (1998). “Temperature effects on fatty acid composition during development of low-linolenic oilseed rape (Brassica napus L.).” Journal of the American Oil Chemists Society, 75 (7) 759-766. doi: 10.1007/s11746-998-0223-4
Publisher – GoogleScholar
8. Dyer, J. M., Stymne, S., Green, A. G., and Carlsson, A. S. (2008). “High-value oils from plants.” Plant Journal, 54 (4) 640-655. doi:10.1111/j.1365-313X.2008.03430.x
Publisher – GoogleScholar
9. Kaluzny, M. A., Duncan, L. A., Merritt, M. V., and Epps, D. E. (1985). “Rapid separation of lipid classes in high yield and purity using bonded phase columns.” Journal of Lipid Research, 26 (1) 135-140.
GoogleScholar
10. Lu, C., Napier, J. A., Clemente, T. E., and Cahoon, E. B. (2011). “New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications.” Current opinion in biotechnology, 22 (2) 252-259.
Publisher – GoogleScholar
11. Meier, M. A., Metzger, J. O., & Schubert, U. S. (2007). “Plant oil renewable resources as green alternatives in polymer science.” Chemical Society Reviews, 36 (11) 1788-1802.
Publisher – GoogleScholar
12. Nosenko, T., Hutsalo, I., Nosenko, V., Levchuk, I., & Litvynchuk, S. (2013). “Analysis of near infrared reflectance spectrum of rape seed with different content of erucic acid”. Ukrainian journal of food science, 1 (1) 94-99.
GoogleScholar
13. Rad, S., and Zandi, P. (2012). “Comparison of winter and spring rapeseed cultivars considering their oil content and fatty acid composition.” American-Eurasian J. Agric. and Environ. Sci. 12 (6) 775-780, doi: 10.5829/idosi.aejaes.2012.12.06.1750
GoogleScholar
14. Rathke, G. W., Christen, O., and Diepenbrock, W. (2005). “Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations.” Field Crops Research, 94 (2—3) 103-113. doi: 10.1016/j.fcr.2004.11.01
GoogleScholar
15. Sas Institute. (2008). SAS software, version 9.2.
16. Schierholt, A., and Becker, H. C. (2011). “Influence of oleic acid content on yield in winter oilseed rape.” Crop Science, 51 (5) 1973-1979.
Publisher – GoogleScholar
17. Sensoz, S., Angın, D., & Yorgun, S. (2000). “Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil.” Biomass and Bioenergy, 19 (4) 271-279.
Publisher – GoogleScholar
18. Suqi, L., Caceres, L., Schieck, K., Booker, C. J., McGarvey, B. M., Yeung, K. & Scott, I. M. (2014). “Insecticidal Activity of Bio-oil from the Pyrolysis of Straw from Brassica spp.” Journal of agricultural and food chemistry, 62 (16) 3610-3618
Publisher – GoogleScholar
19. Venkatachalam, M. and S. K. Sathe (2006). “Chemical Composition of Selected Edible Nut Seeds.” Journal of Agricultural and Food Chemistry 54 (13) 4705-4714.
Publisher – GoogleScholar
20. Wei, F., Yang, M., Zhou, Q., Zheng, C., Peng, J. H., Liu, C. S., and Chen, H. (2012). “Varietal and processing effects on the volatile profile of rapeseed oils.” LWT-Food Science and Technology, 48 (2) 323-329.
Publisher – GoogleScholar
21. Yang, Y., Lu, W., Zhang, X., Xie, W., Cai, M., and Gross, R. A. (2009). “Two-step biocatalytic route to biobased functional polyesters from ω-carboxy fatty acids and diols.” Biomacromolecules, 11 (1) 259-268.
Publisher – GoogleScholar
22. Yu, J. T., Dehkhoda, A. M., & Ellis, N. (2010). “Development of biochar-based catalyst for transesterification of canola oil.” Energy & Fuels, 25 (1) 337-344.
Publisher – GoogleScholar
23. Zheljazkov, V. D., Vick, B., Ebelhar, M. W., Buehring, N., and Astatkie, T. (2012). “Nitrogen applications modify seed and oil yields and fatty acid composition of winter mustard.” Industrial Crops and Products, 36 (1) 28-32.
Publisher – GoogleScholar








