Introduction
Polymorphonuclear neutrophils (PMNs) constitute about 75% of the white blood cells of normal individuals’ peripheral blood and are the major component (>90%) of the granulocytes. They take part in the acute inflammatory response and are the primary immune effector cells against extracellular pathogens. They can be activated by macrophage- and endothelial- derived cytokines. Neutrophils express Fc receptors for IgG and complement receptors and thus bind and phagocytose opsonized antigens, providing an important link between innate and humoral immunity (Pure, 2000). PMN has got special importance in hematological malignancies as the majority of patients of all types show infectious complications associated with neutropenia in AML (Estey et al, 2000), ALL (Dubansky et al, 1989; Benz et al, 2000; Hoelzer, 2000), CML (Crammer et al, 1977), and MDS (Greenberg, 2000). It has been reported that functional activities of PMNs were defective in ALL children undergoing chemotherapy (Lejeune et al, 1998). In addition, some morphological alteration in PMN was also observed in CML and MDS cases. In this connection, it may be noted that both CML and MDS are being regarded as a clonal hematopoietic progenitor cell disorder, and therefore it may be interesting to study the status of PMN in these disorders. Furthermore, it has been established that PMN could be activated for the treatment of B-lymphoblastoid malignancies (Valerius et al, 1997), and is more effective against IL-2 transfected tumor cells (Pericle, 1996) and Rituximab directed CD20+ B-cells (Hernandez-Ilizaliturri, 2003). Evidence also suggests that PMN has direct anti-tumor cytotoxic effect (Koga, 2004). Therefore, it would be interesting to find the HLA status (both class I and II) to get an idea about the pathophysiological state of the first line of defense mechanism of the immune system under the condition.
Materials and Methods
Patients and Normal Individuals
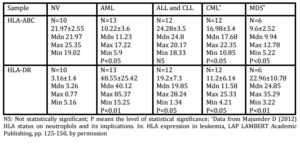
10 ml Peripheral blood (PBL) or 2 ml of bone marrow (BM) samples were collected as per institutional ethical guidelines at the time of diagnosis (de novo & untreated) from the patients suffering from different hematological malignancies. The diagnosis and immunophenotyping categorization of different leukemic patients were done in accordance with the French-American-British (FAB) as mentioned earlier (Majumder et al, 2005; Majumder et al, 2006). Thus, samples of 15 acute myeloid leukemia (AML) (2 of them are M4 and the rest of the samples are either M1 or M2, immunophenotyping characterization also confirms the light microscopic observation), 14 acute lymphoid leukemia (ALL) (all are L1 or L2; immunophenotyping characterization was either CD19+ or CD19+ and CD20+ B lineage cell), 16 chronic myeloid leukemia (CML) (all are in chronic phase and are Ph+), 8 chronic lymphoid leukemia (CLL) (all are either CD19+ or both CD19+ and CD20+ CLL) and 8 myelodysplastic syndrome (MDS) (3 are refractory anemia-MDS, 4 are AML-MDS) were used in this study. Peripheral blood was also collected from 15 normal healthy volunteers (NV) following institutional ethical guidelines.
Processing of Samples
Briefly, collected samples were first diluted about four times and then separated into two compartments by layered preformed Percoll (Sigma, USA) density gradient centrifugation at a density of 1.077 and 1.093 (Pertoft and Lakarent, 1982). Mononuclear cells at 1.077 were removed first, then cells at 1.093 were processed further by using CD15-MACS microbeads and MiniMACS column (Miltenyi Biotec GmbH, Germany) for the enrichment of CD15+ cells (positive panning procedure). Cells isolated at 1.077 were processed further to separate leukemic/immature cells as described earlier (Majumder et al, 2005).
Cell Lines
Raji cell line was obtained from the National Centre for Cell sciences (Pune, India) and was maintained in 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO-BRL, USA). This has been used as a positive control for flow cytometric analysis of HLA-ABC and HLA-DR (Majumder et al, 2005).
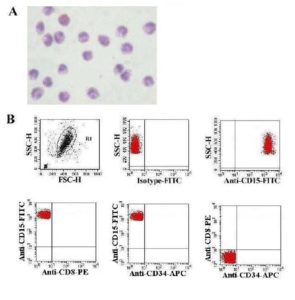
Analysis of Purification of CD15+ Cells by Flow Cytometry
For the evaluation of the purification of neutrophils, enriched cells (as mentioned in processing of samples) were stained with fluorescent isothiocyanate (FITC) conjugated anti-CD15 (BD 340703), phycoerythrin (PE) conjugated anti-CD8 (BD 340046) and allophycocyanin (APC) conjugated anti-CD34 (BD 340667) and subjected to flow cytometric analysis. Cells are gated (G1) first from FSC vs SSC and then analyzed for CD15 vs SSC, CD15 vs CD34 and CD15 vs CD8 by comparing against the corresponding isotype.
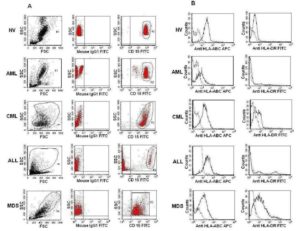
Flow Cytometric Analysis of CD15+ Cell Surface HLA-ABC and HLA-DR
For HLA-ABC and HLA-DR analysis in CD15+ cells’ surface, only fresh samples having more than 20% phenotypic neutrophils in their differential count were used. 100µl of the whole blood was stained with anti-CD15-FITC and anti-HLA-ABC-APC (BD 555555) and analyzed for HLA-ABC expression. Anti-CD15 was used for flow cytometry based phenotypic selection of neutrophils. Similarly, HLA-DR expression analysis on the CD15+ cell 100 ïl of the whole blood was incubated separately with anti-CD15-PE (SC-19595PE) together with anti-HLA DR-FITC (BD 347363) and analyzed by flow cytometer (FACSCalibur, Becton Dickinson, USA). For data analysis, neutrophilic gate (G1) was selected first from FSC and SSC, then from G1 another gate of CD15 vs SSC (G2) was constructed against the corresponding isotype so that only bright cells can be selected (except MDS cases) and the G2 cells were further analyzed for HLA-ABC and HLA-DR expression. Though there is little chance, however, with this method if leukemic blast cells having aberrant CD15 expression are selected then they may slightly skew the observation.
In each case, data of at least 3000-10000 CD15+ cells (depending on the case) were analyzed. FITC, PE, and APC conjugated antibody data were analyzed at Fl1, Fl2 and Fl4 respectively in the flow cytometric analysis. All monoclonals, isotypes, fixation buffer, and FACS Lysing solution were purchased from Becton Dickinson (BD), USA. All antibodies were used as per the manufacturer’s instruction.
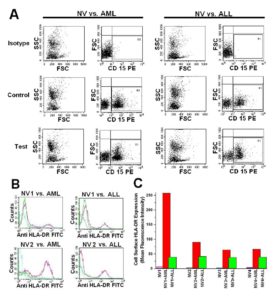
Analysis of HLA-DR Activation on the CD15+ Granulocytes of NV in Presence of Primary Leukemic Cells
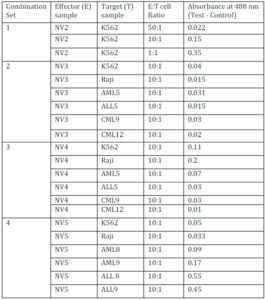
CD15+ granulocytes of NV were mixed with isolated leukemic cells [having immunophenotyping either CD33+ and CD15- (for AML) or CD19+ and CD15- (for ALL)] from leukemic patients in 2 ml of RPMI-1640 and were incubated at 37 ï‚°C with the effector to target cells ratio of 10:1 (Effector = 1ï‚´106 and target = 1ï‚´105 cells) for 20 minutes time interval (Vella et al, 2002). After incubation, cells are labeled with anti-HLA-DR-FITC and anti-CD15-PE (SC-19595PE) and analyzed by flow cytometry. FITC and PE conjugated antibody, data are analyzed at Fl1 and Fl2 respectively in the FACS analysis. CD15+ cells are gated (G1) first from the CD15 vs FSC compared to the isotype and analyzed for HLA-DR expression both in the control (without any target) and in the test (effector with the target) (Figure 3A).
Assessment of CD15+ Granulocytes’ Oxidative Burst Activity Against the Primary Leukemic Cells
To assess the oxidative burst activity (or by ROS production) of the CD15+ granulocytes towards the primary leukemic cells, 5ï‚´106 CD15+ cells of NV were co-cultured with 5ï‚´105 target cells [HLA downregulated leukemic cells: CML (HLA-A+ and -C+, but HLA-B-) and/or CD33+ or CD19+ (both has MFI of HLA-ABC<30)] as well as K-562 and Raji cells] in different tubes (test). The procedure of malignant cells isolation and the assigning of HLA downregulation was described earlier (Majumder et al, 2005). For each set of co-culture reaction, a control tube containing equal number of effector cells and a blank control tube containing equal number of target cells were also taken. To each tube, 200 ïl of 1% NBT (nitroblue tetrazolium, SRL, India) solution was added and volume made up to 3ml with RPMI-1640 and incubated at 37 ï‚°C for 6 hours. After incubation, reaction was stopped by adding chilled 0.1N HCl, centrifuged at 1000g for 15 minutes, supernatant was discarded and the pelleted blue formazone was extracted with equal volume of pyridine (SRL, India) and absorbance of the solution was taken spectrophotometrically at 488 nm (Hudson and Hay, 1993; De Toni et al, 1997; Law et al, 2001). In each of the respective cases, blank control was used to set zero in the spectrophotometer, and killing efficacy was evaluated as the differences in absorbance of the test and the control.
Statistical Analysis
Differences in HLA expression were analyzed by (two-sided) Mann-Whitney U test. A P value less than or equal to 0.05 was considered significant.
The role of immunological surveillance against tumors was largely focused with the lymphocytes and macrophages. Moreover, it has been suggested previously that T cell based immunotherapy will not provide any solution to the HLA downregulated cases. Surprisingly, the functionality of neutrophils against primary leukemic cells has not been documented. Recently, several mechanisms about its functionality have been suggested which include antibody dependent cell cytotoxicity (ADCC) (Elasser et al, 1996; Wurflein et al, 1998), Fas ligand mediated apoptosis (Kim et al, 2000), TNF (tumor necrosis factor) related apoptosis inducing ligand (TRAIL) mediated cytotoxicity (Koga et al, 2004), direct cell killing by phagocytosis followed by intracellular H2O2 and superoxide generation (Lichtenstein and Kahle, 1985). We found that CD15+ cells isolated from NV have a direct killing capacity of HLA downregulated primary leukemic cells, and also have the ability to express HLA class II in presence of primary leukemic cells. In this study, the enhanced HLA-DR expression by CD15+ cells of leukemic patients may be due to the constant cell contact with the malignant cells within the hematopoietic system. This work and our previous work confirm these observations (Majumder, 2012).
It is well established that leukemic patients of all types are more prone to the secondary infection (Estey et al, 2000; Dubansky et al, 1989; Benz et al, 2000; Crammer et al, 1977; Greenberg, 2000). Therefore, CD15+ cells transplantation to leukemic patients (either allogenic or autologous, depending on the case) may have a beneficial role, not only in preventing the secondary infection, but also in killing HLA downregulated primary leukemic cells by ROS production (Hudson and Hay, 1993). It may also be noted that recent clinical trials involving cord blood transplantation in hematological malignancies indicated a better outcome with cells containing a higher neutrophilic counts (Ishikawa and Miyazaki, 2005). Moreover, due to its capacity to express HLA-DR, a long-term benefit of T cell priming by PMNs would be able to provide an added advantage. Thus, as suggested previously (Arcese et al, 2006), the therapeutic application of CD15+ cells may bridge the innate and adaptive immunity in the state of hematological malignancies. Finally, our previous work and that of others (Polakova et al, 2003; Majumder et al, 2006) suggest that innate immune mechanism may be harped in to produce a positive supportive treatment for leukemia.
Acknowledgement
We are grateful to the clinicians and pathologists working in the Ramakrishna Mission Seva Prathisthan, Kothari Medical Center, Kolkata for collecting samples and clinical data. DM acknowledges University Grants Commission, INDIA for providing SRF (NET) Fellowship.
References
1. Arcese W, Rocha V, Labopin M, Sanz G, Lori AP, de Lima M, Sirvent A, Busca A, Asano S, Ionescu I, Wemet P, Glucman E (2006) “Unrelated cord blood transplants in adults with hematologic malignancies,” Haematologica 91:223-230.
Google Scholar
2. Benz SL, Steuber P, Poplack DG (2000) Clinical manifestations of acute lymphoblastic leukemia. In: Hoffman R, Benz Jr. EJ,. Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.), Hematology: Basic principles and practice, 3rd ed., Churchill Livingstone, New York, London, Philadelphia. pp. 1070-1078.
3. Cramer E, Auclair C, Hakim J, Feliu E, Boucherot J, Troube H, Bernard JF, Bergogne E, Boivin P (1977) “Metabolic activity of phagocytosing granulocytes in chronic granulocytic leukemia: ultrastructural observation of a degranulation defect,” Blood 50:93-106.
Google Scholar
4. De Toni S, Piva E, Lapolla A (1997) “Respiratory burst of neutrophils in diabetic patients with periodontal disease,” Annals of New York Academy of Sciences 832:363-367.
Publisher – Google Scholar
5. Dubansky AS, Boyett JM, Falletta J, Mahoney DH, Land VJ, Pullen J, Buchanan G (1989) “Isolated thrombocytopenia in children with acute lymphoblastic leukemia: a rare event in a Pediatric Oncology Group Study,” Pediatrics 84:1068-1071.
Google Scholar
6. Elasser D, Valerius T, Repp R, Weiner GJ, Deo Y, Kalden JR, van de Winkel JG, Stevenson GT, Glennie MJ, Gramatzki M (1996) “HLA class II as potential target antigen on malignant B cells for therapy with bispecific antibodies in combination with granulocyte colony-stimulating factor,” Blood 87:3803-3812.
Google Scholar
7. Enright H, McGlave P (2000) Chronic myelogenous leukemia. In: Hoffman R, Benz Jr. EJ, Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.) Hematology: Basic principles and practice, 3rd ed., Churchill Livingstone, New York, London, Philadelphia. pp 1155 —1171.
8. Estey EH, Kantarjian H, Keating MJ (2000) Therapy for acute myeloid leukemia. In: Hoffman R, Benz Jr. EJ,. Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.), Hematology: Basic principles and practice, 3rd ed., Churchill Livingstone, New York, London, Philadelphia. pp. 1025-1042.
9. Ferron A, Perez-Ayala M, Concha A, Cabrera T, Redondo M, Oliva MR, Ruiz-Cabello F, Garrido F (1989) “MHC class I and II antigens on gastric carcinomas and autologous mucosa,” Journal of Immunogenetics 16:413-423.
Publisher – Google Scholar
10. Gerrard TL, Cohen DJ, Kaplan AM (1981) “Human neutrophil-mediated cytotoxicity to tumor cells,” Journal of National Cancer Institute 66:483-488.
11. Greenberg PL (2000) Myelodysplastic Syndrome. In: Hoffman R, Benz Jr. EJ, Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.) Hematology: Basic principles and practice, 3rd ed., Churchill Livingstone, New York, London, Philadelphia. pp 1106 —1129.
12. Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS (2003) “Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model,” Clinical Cancer Research 9:5866-5873.
Google Scholar
13. Hoelzer D (2000) Acute lymphocytic leukemia in adults. In: Hoffman R, Benz Jr. EJ,. Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.), Hematology: Basic principles and practice, 3rd ed., Churchill Livingstone, New York, London, Philadelphia. pp. 1089-1105.
14. Hudson L, Hay FC (1993) Neutrohil respiratory burst activity. In: Practical Immunology, Blackwell Scientific, Oxford, London, Victoria. pp. 38-42.
15. Ishikawa F, Miyazaki S (2005) “New biodefense strategies by neutrophils,” Archivum Immunologiae et TherapiaeExperimentalis 53:226-233.
Google Scholar
16. Katano M, Torisu M (1982) “Neutrophil-mediated tumor cell destruction in cancer ascites,” Cancer 50:62-68.
Publisher – Google Scholar
17. Kim JM, Kim JS, Jung HC, Song IS, Kim CY (2000) “Apoptosis of human gastric epithelial cells via caspase-3 activation in response to Helicobacter pylori infection: possible involvement of neutrophils through tumor necrosis factor alpha and soluble Fas ligands,” Scandanavium Journal of Gastroenterology 35:40-48.
Publisher – Google Scholar
18. Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T (2004) “Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils,” Cancer Research 64:1037-1043.
Publisher – Google Scholar
19. Law S, Maiti D, Palit A, Majumder D, Basu K, Chaudhuri S, Chaudhuri S (2001) “Facilitation of functional compartmentalization of bone marrow cells in leukemic mice by biological response modifiers: an immunotherapeutic approach,” Immunology Letters 76:145-152.
Publisher – Google Scholar
20. Lejeune M, Ferster A, Cantinieaux B, Sariban E (1998) “Prolonged but reversible neutrophil dysfunctions differentially sensitive to granulocyte colony-stimulating factor in children with acute lymphoblastic leukaemia,” British Journal of Haematology 102:1284-1291.
Publisher – Google Scholar
21. Lichtenstein A, Kahle J (1985) “Anti-tumor effect of inflammatory neutrophils: characteristics of in vivo generation and in vitro tumor cell lysis,” International Journal of Cancer 35:121-127.
Publisher – Google Scholar
22. Majumder D (2012) HLA status on neutrophils and it’s implications. In: HLA expression in leukemia, LAP LAMBERT Academic Publishing, Saarbrucken, pp. 125-150.
23. Majumder D, Bandyopadhyay D, Chandra S, Mukherjee N, Banerjee S (2006) “Lack of HLA-E surface expression is due to deficiency of HLA-E transcripts in the malignant hematopoietic cells of leukemic patients,” Leukemia Research 30:242-5. r
24. Majumder D, Bandyopadhyay D, Chandra S, Mukhopadhayay A, Mukherjee N, Bandyopadhyay SK, Banerjee S (2005) “Analysis of HLA class Ia transcripts in human leukaemias,” Immunogenetics 57:579-589.
Publisher – Google Scholar
25. Pericle F, Kirken RA, Epling-Burnette PK, Blanchard DK, Djeu JY (1996) “Direct killing of interleukin-2-transfected tumor cells by human neutrophils,” International Journal of Cancer 66:367-373.
Publisher – Google Scholar
26. Pertoft H, Lakarent P (1982) Sedimentation of cells in colloidal silica (Percoll). In: Pretlow TG, Tretlow TP (Eds.) Cell separation — Methods and selected application, Vol. I. Academic Press, USA. pp 115-152.
27. Pickaver AH, Ratcliffe NA, Williams AE (1972) “Cytotoxic effects of peritoneal neutrophils on a syngenic rat tumour,” Nature New Biology 235:186-187.
Google Scholar
28. Polakova K, Krcova M, Kuba D, Russ G (2003) “Analysis of HLA-G expression in malignant hematopoetic cells from leukemia patients,” Leukemia Research 27:643-648.
Publisher – Google Scholar
29. Pure E (2000) Overview of the immune system (Including compartmentalization of the immune response), In: Hoffman R, Benz Jr. EJ, Shantil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (Eds.) Hematology: Basic principles and practice, 3rd ed. Churchill Livingstone, New York, London, Philadelphia, pp. 74-79.
30. Valerius T, Elsasser D, Repp R, Van de Winkel JG, Gramatzki M, Glennie M (1997) “HLA class II antibodies recruit G-CSF activated neutrophils for treatment of B cell malignancies,” Leukemia Lymphoma 26:261-269.
Google Scholar
31. Vella A, Sartoris S, Bambara L (2002) “Cell contact-dependent PMN HLA-DR and CD69 membrane expression induced by autologous mono-lymphocytes and cell lines,” Inflammation 26:143-152.
Publisher – Google Scholar
32. Wurflein D, Dechant M, Stockmeyer B, Tutt AL, Hu P, Repp R, Kalden JR, van de Winkel JG, Epstein AL, Valerius T, Glennie M, Gramatzki M (1998) “Evaluating antibodies for their capacity to induce cell-mediated lysis of malignant B cells,” Cancer Research 58:3051-3058.
Google Scholar
33. Zivokovic M, Poljak-Blazi M, Egger G, Sunjic SB, Schaur RJ, Zarkovic N (2005) “Oxidative burst and anticancer activities of rat neutrophils,” Biofactors 24:305-312.
Publisher – Google Scholar