Introduction
Burkitt’s lymphoma (BL) is a peripheral B-cell lymphoproliferative malignancy.
BL is quite rare outside of Africa, uncommon in North America and in Europe, occurs in pediatric population, and is one of the fastest-growing malignancies in the early- ages. The endemic form has a good prognosis and the association with HIV infection is frequent (1).
Disease confined to abdomen is seen in 50% of cases at presentation, most of them with bowel or mesenteric tumors (2). Extranodal presentation of BL is rare. Although diplopia and ocular palsies have been previously reported in patient with BL, rarely bilateral cavernous syndrome is the initial manifestation in immunocompetent adults. Previous reports desdribed B L of the skull base with invasion of the cavernous sinus (CS) especially in children (3 – 5). Four cases of CS initial presentation of sporadic BL have been recently described (6 – 9). We report an additionnal case presenting with unusual features.
Case Report
A 42-year-old woman without ongoing health problems developed within two weeks severe right orbital pain, associated with nausea, phonophotophobia, sweats, poor appetite, weight loss and lassitude. Two days later, she presented with a diplopia and a left ptosis. Neurological examination revealed incomplete palsy of the left III and right VI nerves, and sensory loss of the first and second division of the left trigeminal nerve. Physical examination revealed two breast masses of 10 cm of diameter and multiple axillary nodes, bilaterally.
Laboratory studies showed a hemoglobin count of 9,6 g/dl, normal white blood cells and platelet count, and a marked elevation in the level of lactate dehydrogenase (LDH) ( 1364 IU/L), which suggested the presence of tumor lysis syndrome. Other blood biochemical parameters were normal and there was no evidence of HIV infection. CSF biochemistry and cyto-analysis were unremarkable.
CT and MRI of the brain demonstrated masses in the CS. On CT, the cavernous sinuses appeared enlarged and enhanced after contrast and there was no evidence of skull base or sellar destruction or erosion. On MR, there was symmetrical enlargement of both cavernous sinuses. The masses appeared isointense to the cortex on -T1- and T2-weighted images and FLAIR sequence, and enhanced slightly and homogeneously after gadolinium (Fig. 1-a-d). The cavernous sinus infiltration did not extend into the contiguous regions. Diffusion-weighted images (DWI) showed low signal intensity CS masses (Fig 1- e) and low ADC values. Magnetic reconance angiography (MRA) demonstrated displaced internal carotid arteries in their CS course (Fig 1-f). Additionnal tumor work-up showed on CT large pelvic mass with lymph nodes enlargement. Biopsy of the left breast nodule revealed BL. Infiltration consisted mainly of lymphoïd cells with basophil cytoplasm, round or ovalar nucleus and many mitotic figures. Immunohistochemistry showed strong positivity for the B cell markers CD20, CD79A, CD10. Bone marrow aspiration biopsy disclosed massive proliferation of B great cells with positivity for CD20, CD10 cell markers and Epstein-Barr EBER-1 was detected immunohistochemically. On the basis of clinical, radiologic, cytologic, and immunophenotypic results, BL’s stage IV with important bulk tumor was diagnosed, and chemotherapy type cyclophosphamide-vincristine regimen was initiated.
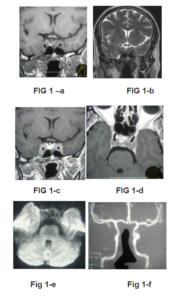
Fig 1 —MRI at Initial Presentation
— Coronal T1- (a) and T2-weighted images (b) show symetrical enlargement of the cavernous sinuses by the lesion which has an homogeneous and isointense signal intensity to the cortex and complete encasement of the internal carotid artery (ICA) by the tumor.
– c. Coronal (same plane than Fig 1-b) and Axial (d) contrast-enhanced T1-weignted image shows slight and homogeneous enhancement of the masses.
– e- Diffusion-weighted image (b= 1000s/mm²) shows low signal from the CS masses.
– f — MRA shows abnormal ICAs in their CS courses
Brain and diffuse meningeal involvement occured six months later and the patients died 11months after the initialization of treatment.
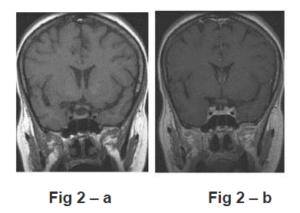
Fig 2 — MR follow-up, 3 Weeks after Initialization of Chemotherapy
Coronal T1 (a) and T1 after contrast-enhancement (b) images reveal the complete disappeareance of the lesion. Both cavernous sinuses are free of tumoral invasion.
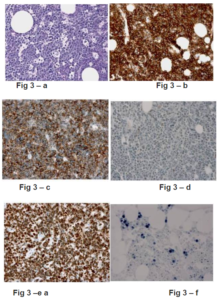
Fig 3-a to 3-b: Biopsy of the Left Breast Nodule
Histologically, Burkitt lymphoma is composed of uniform cells with small nucleoli and dispersed chromatin (3-a); tumor cells strongly express CD20 (3-b), CD10 (3-c) with a proliferation index of 100% with Ki67 (3-e).
Fig 3-f: Bone Marrow Biopsy
Sparced Burkitt lymphoma tumor cells are positive for EBER-1 situ hybridization
Discussion
More than 90% of primary Central Nervous System (CNS) lymphomas are histologically classified as high-grade B cell lymphomas and about 5-15% of these are of Burkitt’s type (10). BL is charactherized by rapid progression and early hematogeneous dissemination.
EBV viral antigens have been found in 50% to 90% of patients and are uncommon in the “sporadic form” (11). In the presented case, EBV antigen was detected immunochemically in B marrow cells.
Initial presentation with CNS or skull base involvement is a rare event, occurring more frequently in non-African form, and is associated with the greatest risk of treatment failure. CS invasion as an initial presentation of BL has been reported only rarely. The clinical signs are those of a cavernous sinus syndrome (CSS), characterized by ocular motor nerves palsies involving the third, fourth and sixth nerves, as well as the first or second divisions of the fifth cranial sensory nerve in various combinations, and orbital pain. The pupil may be involved or spared (12). MRI findings may be useful for diagnosis (13).
Solitary primary BL of the CS has been reported in two cases and diagnosis was achieved by surgical biopsy of the lesion (6, 8) and bilateral and asymetrical invasion of the CS as the initial presentation has been reported in another case (7, 9). Rare are the reports on MRI findings of BL of the CS, an appearance which is characterized by an isointensity to the brain on T1- and T2-weighted images. On post-contrast T1-weighted images, enhancement is always homogeneous, usually slight, as in our case. Rarely post-contrast enhancement is intense (6, 7). These radiological features are not specific, and slight enhancement is suggestive of lymphomas. Furthermore, low-signal on DWI is rarely observed in solid and enhancing intracranial tumors, especially intraaxial tumors. We could find no relevant report of DWI in cavernous sinuses lymphomas.
Our case is unusual by the bilateral and symmetrical CS involvement, the occurence in a immunocompetent non-African woman and the association to EBV antigenicity.
Patients with increased tumor bulk and widely disseminated diseases have lower response rates as in our case.
Numerous causes for CSS have been reported and fall into categories of tumors (30%), infectious and inflammatory lesions, as well as vascular lesions. Lymphomas constitute 6% of all lesions (12).
Bilateral CS involvement suggests especially lymphomas, and infectious diseases, more rarely CS fistulas. More common infectious lesions involving the CS are aspergillomas or mucormycosis. They occur in immunocompromised and diabetic patients and arise as a result of extension from the paranasal sinuses, and orbital involvement is usually associated. Thrombosis of the CS may also be bilateral, complicating bacterial or fungal invasion of the sinuses and imaging consists in lack of enhancement inside the CS and increased enhancement along the lateral border of the CS. In these cases of CSS, CT and MR provide useful information to the diagnosis. Bilateral CS tuberculomas are very uncommon and are associated with the enhancement of the adjacent thickened dura (14). Non-infectious inflammatory lesions such as sarcoidosis have also to be considered.
Metastatic disease of the CS is not an uncommon cause of CSS. Its presentation, however, occurs almost exclusively as a unilateral, rapidly progressive painful ophtalmoplegia and is an extremely rare cause of bilateral CS involvement ( 9; 15 ). Carotid cavernous fistulas cause also CSS, may be bilateral, and are associated with particular orbital congestion and bruit. Presence of signal voids inside the CS on T2-weighted images is a very specific feature (16). In these CS lesions, MRI may be useful for diagnosis (13).
Meningioma remains the most frequent tumor in this location. CS meningioma is associated with the enhancement of the dural attachment and in only rare cases invades the sellae and both cavernous sinuses. When the enhancement is not typical for a meningioma, a work-up for lymphomas must be realized.
In CSS in relation to BL, biopsy of associated locations usually allow the diagnosis as in the literature and herein reported case ( 7).When cavernous sinus lesion is solitary, surgical biopsy of this location is necessary (6)
Conclusion
Primary CNS involvement of Burkitt Lymphoma is rare, occurs more frequently in sporadic form and cavernous sinus involvement as initial presentation is uncommon.
Burkitt Lymphoma should be considered in the differential diagnosis and a work-up of the tumor must be realized when cavernous sinus enlargement is present at MRI with the herein described signal.
Since BL has the higher growth rate of any tumor, rapid diagnosis and immediate treatment are important and likely to improve outcome.
References
1 — Spina, M., Tirelli, U., Zagonel, V., et al. (1998). “Burkitt’s Lymphoma in Adults with and without Human Immunodeficiency Virus Infection,” Cancer; 82 (4): 766-774
Publisher – Google Scholar
2 — Johnson, K. A., Tung, K., Mead, G., et al. (1998). “The Imaging of Burkitt’s and Burkitt-Like Lymphoma,” Clinical Radiology; 53 (11): 835-841.
Publisher – Google Scholar
3 — Delpassand, E., Kirkpatrick, J. (1988). “Cavernous Sinus Syndrome As the Presentation of Malignant Lymphoma : Case Report and Review of the Literature,” Neurosurgery ; 23 (4) : 501-503.
Publisher – Google Scholar
4 — Ceyhan, P., Erdem, G., Kanra G, et al “Lymphoma with Bilateral Cavernous Sinus Involvement in Early Childhood,”Pediatric Neurology; 10 (1) : 67-69.
Publisher – Google Scholar
5 — Kalina, P., Black, K., Woldenberg, R. (1996). “Burkitt’s Lymphoma of the Skull Base Presenting as Cavernous Sinus Syndrome in Early Childhood,” Pediatric Radiology.; 26(6):416-417.
Publisher – Google Scholar
6 — Dufour, H., Diaz, A., Metellus, P. et al. (2001). “Lymphome De Burkitt Du Sinus Caverneux. A Propos D’un Cas,”Neurochirurgie; 47 (6): 564-567.
Publisher – Google Scholar
7 — Huisman, T.A., Tschirch, F., Schneider, J. F. et al. (2003). “Burkitt’s Lymphoma with Bilateral Cavernous Sinus and Mediastinal Involvement,” Pediatric Radiology. Oct;33(10):719-21
Publisher – Google Scholar
Publisher – Google Scholar
Publisher – Google Scholar
10 — Jellinger, K. A., Paulus, W. (1992). “Primary Central Nervous System Lymphomas,” Journal of Cancer Research and Clinical Oncology; 119 (1): 7-27.
Publisher – Google Scholar
11 – Brengel-Pesce, K., Morand, P., Schmuck, A. et al. (2002). “Routine Use of Real-Time Quantitative PCR for Laboratory Diagnosis of Epstein-Barr virus infections,” Journal of Medical Virology;66 (3): 360-369
Publisher – Google Scholar
12 — Keane, J.R. (1996). “Cavernous Sinus Syndrome,” Archives of Neurology; 53 (10): 967-971.
Publisher – Google Scholar
13 — Lee, J.H, Lee, H.K, Park, J.K. et al. (2003). “Cavernous Sinus Syndrome: Clinical Features and Differential Diagnosis with MR Imaging,” American Journal of Roentgenology;181 (2): 583-590.
Publisher – Google Scholar
14 — Grayeli, A. B., Redondo, A., Salama, J, et al. (1988). “Tuberculoma of the Cavernous Sinus : Case Report,”Neurosurgery; 42: 179-182.
Publisher – Google Scholar
15 — Supler, M. & Friedman, W.A. (1992). “Acute Bilateral Ophtalmoplegia Secondary to Cavernous Sinus Metastasis: A Case Report,” Neurosurgery.;31 (4): 783-786.
Publisher – Google Scholar
16 – Stiebel-Kalish, H., Setton, A., Berenstein A et al. (2002). “Bilateral Orbital Signs Predict Cortical Venors Drainage tn Rhe Cavernous Sinus Dural AVMs,” Neurology.;50 (10): 1521-1524.
Publisher – Google Scholar






