Introduction
Hepatitis C virus (HCV) infection and alcohol are the two most common causes of liver disease in the United States (US) (Anonymous 2002). It is well established that alcoholics with HCV infection develop cirrhosis and hepatocellular carcinoma more frequently than alcoholics without HCV infection (Peters and Terrault 2002; A. K. Singal and Anand , 2007). However, there is limited data on the effect of CHC on the outcome of alcoholic hepatitis (AH). We conducted the present study using the National Inpatient Sample (NIS) dataset to examine the impact of HCV on the in-hospital mortality in patients with AH. The study was approved by the Institutional review board.
Methods
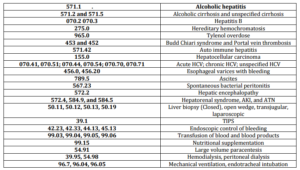
The Nationwide Inpatient Sample (NIS) dataset with details of about 8 million hospital discharges (with up to 15 diagnosis and 15 procedure codes) from over 1,050 hospitals in 42 states in the US. Discharges with a primary or secondary diagnosis of AH (ICD-9-CM code 571.1) were stratified based on their HCV status (ICD-9-CM code 070.41, 070.44, 070.51, 070.54, 070.70, 070.71). The primary outcome measure was in-hospital mortality. Two groups of AH patients (HCV +ve and HCV —ve) were compared with respect to patient demographics, Charlston Comorbidity Index (CCI) (Quan et al. 2005), admission type (elective vs. emergency), pay source (self employed or medicaid payment), hospital characteristics (urban or rural, teaching or non-teaching, bed size and geographical location), length of hospital stay, liver disease complications, diagnostic or therapeutic interventions, and outcome. Specific codes were used to identify each of these variables in the dataset (Table 1).
Table 1. List of International Classification of Diseases (ICD-09) Codes Used for the Analysis
Continuous variables were compared using t-test and categorical variables using Chi-square test. Logistic regression model was built to study the independent association of HCV and in-hospital mortality. Variables which could have confounded the outcome were entered into the model. The results of logistic regression were described as odds ratio (OR) with 95% confidence interval (95% CI).
Results
A total of 111,726 AH admissions (6.5% with concomitant HCV infection) between 1998 and 2007 were analyzed. Patients with AH and concomitant HCV infection were younger, required admission on an emergent basis, were of black or Hispanic ethnicity, had a higher CCI score, were on Medicaid pay source, were more likely to be in a large, urban teaching hospital, located in the Western region of the country, and more frequently had liver related complications (Table 2).
Table 2. Characteristics of Patients with Alcoholic Hepatitis (1998-2007): Comparison of HCV Positive and HCV Negative Cases
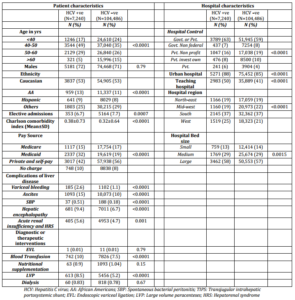
Although, overall mean length of hospital stay was similar between HCV positive and HCV negative groups (6.0±5.8 vs. 5.5±39 days; P=0.28), the proportion of patients staying in the hospital for more than 7 days was higher in HCV +ve AH admissions (23% vs. 21%; P<0.0001).
A total of 3,541 (3.2%) patients died (higher with HCV: 3.8% vs. 3.1%; P=0.001) during the hospital stay. In-hospital mortality decreased at about 7% per year [OR 0.93 (CI: 0.91-0.94; P<0.0001)] over the last decade. After controlling for other confounding variables including the calendar year, HCV was an independent predictor of in-hospital mortality in patients admitted with AH (Table 3).
Table 3. Logistic Regression Model to Determine Independent Prediction of HCV on the in-Hospital Mortality of Patients with Acute Alcoholic Hepatitis
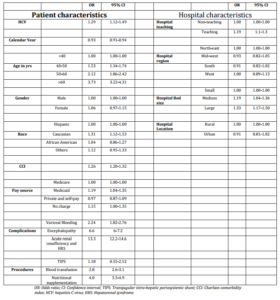
Other predictors of in-hospital mortality were age at admission, Caucasian race, Medicaid pay source, CCI score, admission in a teaching hospital, complications from liver disease (renal failure, hepatic encephalopathy, and variceal bleeding), and the need for diagnostic or therapeutic interventions.
Discussion
Although, AH is an acute condition, concomitant cirrhosis may be present in many patients (O’Shea et al.). The clinical presentation of worsening cirrhosis may mimic AH if the clinical or laboratory evidence of systemic inflammation such as fever, chills, tender hepatomegaly and neutrophilic leucocytosis are lacking. Therefore, we excluded admissions from the study population with a primary diagnosis of cirrhosis.
Patients with AH and concomitant HCV infection had worse outcome with a higher in-hospital mortality. This is reflected with higher rate of complications amongst patients with concomitant HCV infection. Several studies in the past have assessed the impact of HCV on alcoholic cirrhosis. However, the effect of HCV on the outcome of AH has not been examined properly. A previous study observed that patients with AH had a worse outcome at 6 months follow-up in the presence of HCV infection compared to patients without HCV (A. S. Singal, S; Kuo, YF; Weinman, SA. 2011). However, this was a small retrospective study from a single institution.
AH is a serious medical condition with a mortality of about 40% at 6 months in patients with severe disease despite treatment with corticosteroids and/or pentoxifylline (Lucey et al. 2009). In the present study, the overall in-hospital mortality was only 3.2%. Being a data set analysis, rigorous clinical criteria for the diagnosis of AH can’t be applied. It is possible that this may have resulted in a more heterogeneous data collection and included patients with relatively milder forms of disease. The lack of availability of laboratory parameters in this data set limits determination of disease severity. However, we believe that the large sample size from a nationally representative database should overcome this limitation to a great extent. Since the number of admissions was the denominator for estimating the mortality, this may also have falsely lowered the inpatient mortality from AH as a patient may have been admitted several times prior to mortality. Furthermore, the lack of follow-up data after discharge from the hospital, limits the long-term outcome analysis of these patients.
In summary, our study indicates that in-hospital mortality of patients admitted with AH is low. However, the presence of concomitant HCV infection is associated with higher in-hospital mortality. If these findings are confirmed in prospective studies and from databases with outpatient follow up, HCV infection in patients admitted with AH may be incorporated into scoring systems designed for the assessment of disease severity and mortality.
References
Anonymous (2002). “National Institutes of Health Consensus Development Conference Statement Management of Hepatitis C: 2002–June 10-12, 2002,” Hepatology, 36 (Suppl 5), S3-S20.
Publisher – Google Scholar
Lucey, M. R., Mathurin, P. & Morgan, T. R. (2009). “Alcoholic Hepatitis,” N Engl J Med, 360 (26), 2758-69.
Publisher – Google Scholar
O’Shea, R. S., Dasarathy, S. & McCullough, A. J. (2010). “Alcoholic Liver Disease,” Am J Gastroenterol, 105 (1), 14-32.
Publisher – Google Scholar
Peters, M. G. & Terrault, N. A. (2002). “Alcohol Use and Hepatitis C,” Hepatology, 36 (5 Suppl 1), S220-5.
Publisher – Google Scholar – British Library Direct
Quan, H., Sundararajan, V., Halfon, P., Fong, A., Burnand, B., Luthi, J.- C., Saunders, L. D., Beck, C. A., Feasby, T. E. & Ghali, W. A. (2005). “Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data,” Med Care, 43 (11), 1130-9.
Publisher – Google Scholar – British Library Direct
Singal, A. K. & Anand, B. S. (2007). “Mechanisms of Synergy between Alcohol and Hepatitis C Virus,” J Clin Gastroenterol, 41 (8), 761-72.
Publisher – Google Scholar – British Library Direct
Singal, A. K., Sagi, S., Kuo, Y.- F. & Weinman, S. (2011). “Impact of Hepatitis C on the Outcome and Severity of Acute Alcoholic Hepatitis,” Eur J Gastroenterol Hepatol, 23 (3), 204-9.
Publisher – Google Scholar





