Introduction
Chitin is a polysaccharide of animal origin found abundantly in nature and characterized by a fibrous structure. It forms the basis of the main constituent of the outer skeleton of insects and crustaceans like shrimp, crabs and lobster (Kumar et al., 2005). According to Chen (1998) the chitin structure can be modified by removing the acetyl groups, which are bond to amine radicals in the C2 position on the glucan ring, by means of a chemical hydrolysis in concentrated alkaline solution at elevated temperature to produce a deacetylated form known as chitosan.
No, H.K. et al., (2002) stated that antibacterial activity of chitosan is effective in inhibiting growth of bacteria. The antimicrobial properties of chitosan depend on its molecular weight and the type of bacterium. For gram-positive bacteria, chitosan with 470 KDa was the most effective, except for Lactbacillus sp., whereas for gram-negative bacteria, chitosan with 1,106 KDa was effective. Chitosan generally showed stronger bactericidal effects for gram positive bacteria (Listeria monocytogenes, Bacillus megaterium, B. cereus, Staphylococcus aureus, Lactobacillus plantarum, L. brevis, and L. bulgaris) than for gram-negative bacteria (E.coli, Pseudomonas fluorescens, Salmonella typhymurium, and Vibrio parahaemolyticus) in the presence of 0.1% chitosan. The antimicrobial activity of chitosan is described to be associated with molecular weight, degree of acetylation, concentration of chitosan and bacterial inoculum size was showed by Chen, M.C et al., (1996) and Fernandes, J.C et al., (2008). It is reported by Peter E. J. C. et al. (2008) that lower molecular weight chitosan is more effective against Gram-negative bacteria, whereas high molecular weight chitosan is effective against Gram-positive bacteria in atomic force microscopy of cell wall structure and nano-indentation study. In this research work we tried to show the activity of chitin and chitosan against two pathogenic bacteria, one gram positive strainstaphylococcus aureus and one gram negative strain Escherichia coli. These pathogenic bacteria are all around us contaminating our food, water readily, causing spoilage of perishable food items. The bacterial effectiveness on gram-positive or gram-negative bacteria is however, somewhat controversial. Some authors have stated that chitosan generally showed stronger effects for gram-positive bacteria (e.g. Listeria monocytogenes, Bacillus megaterium, B. cereus, Staphylococcus aureus, Lactobacillus plantarum, L. brevis, L. bulgaris, etc.) than for gram-negative bacteria (e.g. E. coli, Pseudomonas fluorescens, Salmonella typhymurium, Vibrio parahaemolyticus, etc.) Coma et al, Jeon et al. and No et al. (2001-03), and Dutta (2009). Conversely, it has been demonstrated that hydrophilicity in gram-negative bacteria is significantly higher than in gram-positive bacteria, making them most sensitive to chitosan stated by Chung et al., (2004). The charge density on the cell surface is a determinant factor to establish the amount of adsorbed chitosan. More adsorbed chitosan would evidently result in greater changes in the structure and in the permeability of the cell membrane. This would suggest that the antibacterial mode of action is dependent upon the host microorganism Másson, M. et al. (2008).
The antimicrobial activity of chitosan is described to be associated with molecular weight, degree of acetylation, concentration of chitosan and bacterial inoculum size was described by Chen M.C. et al 1996, Fernades J.C et al. (2008). It is reported that lower molecular weight chitosan is more effective against Gram-negative bacteria, whereas high molecular weight chitosan is effective against Gram-positive bacteria in atomic force microscopy of cell wall structure and nano-indentation study by Peter E.J.C et al. (2008).
In this research work we tried to show the activity of chitin and chitosan against two pathogenic bacteria, one gram positive strain staphylococcus aureus and one gram negative strain Escherichia coli.
The result obtained from the research work will contribute to evolve a more active natural antibacterial agent which may be applied to preserve food items, may be used as antimicrobial agent in pharmaceuticals, and may also find uses in agriculture as a more active Seed coating, fertilizer, controlled agrochemical release agent. In drug industry it may be used to formulate weight loosing supplement, which is already produced in many countries. As Bangladesh is an agro based country we think production of chitin and chitosan will confined into variety of uses. Chitosan production from shrimp waste certainly save us from serious environment pollution, we should use this biopolymer as a safe preservative in food item, also can be apply as seed coatings, fertilizer, we are with serious water problem in municipal area, chitosan may be used as water purification agent.
Based on the above investigation the following objectives were undertaken:
- The study investigates chitosan (produced by chemical deacylation) and chitin produced exclusively from shrimp of our country (as region or source may affect activity) whether possess antibacterial activity and
- To compare whether chitin is more active than chitosan and vice-versa against E. coli and Staphylococci.
Methodology
Chitin is extracted from crustacean shell waste such as crab, shrimp, lobster, and crawfish. We prepared chitin from shrimp shell (Macrobrachium rosenbergii) locally known as Galda chinri. Isolation of chitosan from shirmp shell wastes involves four traditional steps demineralization (DM), deproteinization (DP), decolorization (DC), and deacetylation (DA). the method was modified from No and Meyers, (1995). Two bacterial strains were tested for the antimicrobial activity of chitosan and chitin include gram negative bacteria Escherichia coli gram positive Staphylococcus aureus (pure culture was obtained from Sono Diagonostic & hospital, Kushtia) pure Staphylococci culture was procured from Food microbiology research lab-2, Islamic university, kushtia). Antibacterial susceptibility test (AST) was used to determine the efficiency of potential antimicrobials and initial inoculums bacterial count was made. AST methods are used to screen antimicrobial activity but are largely used to determine usefulness of an antimicrobial to combat against infections. The current study was carried out by broth dilution method, since it is most economical method for AST. In this exercise we used turbidity estimations to determine the growth characteristics of a bacterial culture. For this we first ensure same characteristics of the inoculation medium. The sequence of procedure to accomplish this is as follows–
Growth Media: The sterile growth media was poured into 18 sterile test tubes in aseptic condition using laminar air flow cabinet. In every test tube 9ml of nutrient broth media was taken. Few test tubes will be used as standard in spectrophotometer measurement.
Addition of Chitin and Chitosan: 3% (v/v) 100 ml acetic acid preparation: 3ml concentrated (99%) acetic acid was taken into a conical flask and made up to 100ml volume mark by distill water.
1.5gm chitin and chitosan was taken into two test tube (sterile) and 10ml 3% acetic acid was poured into it gradually. To increase the solubility the solution stirred and heat was also applied in water bath at 40º C. The solution of chitin and chitosan was then added into the test tubes. The upper soluble portion of each sample was added with medium, we did not take the supernatant from the test tube. In 6 test tubes 0.5 ml chitosan (in each test tube) solution was taken by micropipette. Similarly another test tube was prepared with chitin solution (0.5ml in each test tube). Both for chitin and chitosan, from these 6 test tubes one was used as standard (media+ chitin / media+ chitosan), one for negative control (media+ chitin / media+ chitosan), two for gram negative bacteria inoculation (media+ chitin+ gram negative / media+ chitosan+ gram negative), and two for gram positive bacteria inoculation( media+ chitin+ gram positive / media+ chitosan+ gram positive). Of the remaining 6 test tubes one was used standard (only media), one for negative control (only media), two for gram negative inoculation (media+ gram negative), and two for gram positive inoculation (media+ gram negative).
pH Control: We adjusted the pH of the medium. The pH of only medium was 6.5. The pH of chitin with medium, chitosan with medium was also adjusted between 6.2-6.5 range, using NaOH solution. All these were done in laminar air flow cabinet.
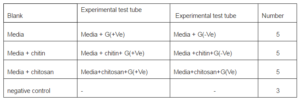
Inoculation of Bacteria: After adjustment of the pH we inoculated the test tubes, with gram positive staphylococcus aureus and gram negative Escherichia coli. The following table shows the number of test tubes inoculated, with G (+Ve) and G (-Ve) bacteria respectively. The experiment was conducted in a view to compare the growth of bacterial strain in only media, and in media with chitin and chitosan.
Table 1. Shows the Number Pattern of Inoculation of Test Tubes
Incubation: After successful inoculation we incubated the test tubes in an incubator, and the temperature was set at 37oC. After each 4 hours later we took the turbidimetric measurement by spectrophotometer.
Turbidimetric Measurement of Bacterial Growth: Growth is commonly measured by determining the turbidity of a cell suspension with an instrument known as a spectrophotometer or colorimeter. In this exercise we used turbidity estimations to determine the growth characteristics of a bacterial culture. In liquid culture, the medium appears more and cloudier as the bacteria increase in number by division. A tube of bacteria will tend to reflect light so that less light is transmitted through the tube. A spectrophotometer can measure the amount of light passing through the tube, or conversely the amount of light absorbed. These measurements of turbidity or optical density (OD) are not direct measurements of bacterial numbers, but an indirect measurement of cell biomass that includes both living and dead cells. As the bacterial cell population increases, the amount of transmitted light decreases, increasing the absorbance reading on the spectrophotometer. If one takes readings of the same culture over time, the absorbance readings will increase as the cell number increases.
Results and Discussions
If the turbidity measured is greater, in case of medium and inoculum (measured in terms of medium as standard) than turbidity of medium chitin and inoculum (measured in terms of medium and chitin as standard) or medium chitosan and inoculum (measured in terms of medium and chitosan as standard), then we can conclude that chitin or chitosan exhibit antibacterial property. The result was interpreted numerically and graphically to demonstrate comparative antibacterial activity of chitosan and chitin.
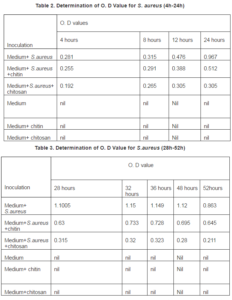
In every O.D value measured staphylococcus aureus with chitin gave far higher absorbance than chitosan. It is clearly evident that chitosan possess high level antibacterial property against S.aureus, while chitin possess lower level of activity than chitosan. At the end of 32 hour’s incubation the peak or highest growth for S. aureus and the absorbance was 1.15, while in chitosan containing S.aureus medium absorbance was 0.32, and chitin containing S.aureus medium absorbance was 0.733. We did not find any peak for chitosan containing medium sample as found in normal growth curve. Chitosan could not completely inhibit the growth of S.aureus this is because we did not take the minimum inhibitory concentration (MIC) concentration of chitosan. The data was presented graphically below:
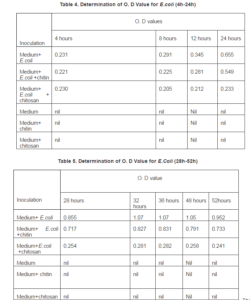
The O.D value for E.coli clearly demonstrate antibacterial activity against gram negative E.coli. After 36hours of inoculation the maximum growth for the inoculum and the Absorbance was 1.07, while chitosan with E.coli ABS was 0.282, and chitin with E.coli Absorbance was .831. Chitosan shows strong activity against E.coli, while chitin has much lower activity. The data graphically below:
Comparative Study:
Chitosan possess strong antibacterial activity against both G (+Ve) and G (-Ve) bacteria, while chitin possess lower level of activity against these two types of bacteria. The result was numerically presented to determine which types of bacteria is mostly affected by chitosan and chitin:
Comparative Study of Antibacterial Activity against G (+Ve) and G (-Ve) of Chitosan
The absorbance for medium and S.aureus at the peak was 1.15. While the absorbance for medium and S.aureus+chitosan at the peak was 32. The ratio between these two absorbance is 0.28:1. So the ratio of medium and S.aureus+chitosan: medium + S.aureus = 0.28:1.
The absorbance for medium and E.coli at the peak was 1.07, while the absorbance for medium and E.coli +chitosan at the peak was 0.282. The ratio between these two absorbance 0.26:1. So the ratio of medium and E.coli + chitosan : medium + E.coli =0.26:1.
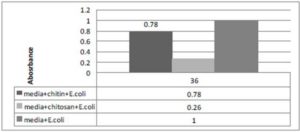
Figure 1. Ratio between Medium E.coli and Chitin or Chitosan with Medium and E.coli.
The figure represent 1 unit absorbance for media and E.coli at the same time or same hour of incubation E.coli with chitosan absorbance found 0.26 and chitin with E.coli recorded 0.78. So chitosan is about 3.0 times more active againstE.coli than chitin.
Comparative Study of Chitosan and Chitin against G (-Ve) Bacteria:
Now we compared numerically the activity of chitosan and chitin against G (-Ve) E.coli, we used the obtained at the peak. The absorbance for medium and E.coli at the peak was 1.07 while the absorbance for medium and E.coli +chitosan at the peak was 0.311. The ratio between these two absorbance = 26:1. So the ratio of medium and E.coli +chitosan: medium +E.coli = .26:1. The absorbance for medium and E.coli at the peak was 1.07 while the absorbance for medium and E.coli+chitin at the peak was .831. The ratio between these two absorbance = 0.78. So the ratio of medium and E.coli +chitin: medium + E.coli = 0.78:1. So chitosan is 0.78/0.26=3.0 times more active than chitin against E.coli.
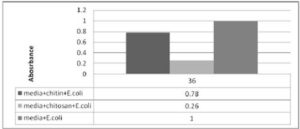
Figure 2. Shows the Ratio between Medium E.coli and Chitin or Chitosan with Medium and E.coli.
The figure represent 1 unit absorbance for media and E.coli at the same time or same hour of incubation E.coli with chitosan absorbance found 0.26 and chitin with E.coli recorded 0.78. So chitosan is about 3.0 times more active againstE.coli than chitin.
Comparative Study of Chitosan and Chitin against Gram Positive Bacteria:
The absorbance for medium and S.aureus at the peak was 1.15 while the absorbance for medium and S.aureus + chitosan at the peak was 0.32. The ratio between these absorbance is 0.28:1. The ratio of medium and S.aureus+chitosan: medium + S.aureus = 0.28:1. The absorbance for medium and S.aureus at the peak was 1.15 while the absorbance for medium and +chitin at the peak was 0.733. The ratio between these two Absorbance= 0.64. So the ratio of medium and S.aureus +chitin: medium + S.aureus= 0.64:1 chitosan is 0.64/ 0.28= 2.2 times more active than chitin against S. aureus.
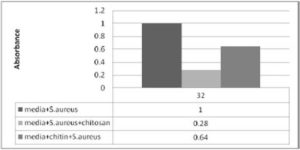
Figure 3. Shows the Ratio between Medium S.aureus and Chitin or Chitosan with Medium and S.aureus.
The figure represent 1 unit absorbance for medium and S. aureus at the same time or same hour of incubation S. aureuswith chitosan found 0.28 and chitin with S. aureus recorded 0.64. So chitosan is about 2.2 times more active againstS.aureus than chitin. The chitin and chitosan was found to be bacteriostatic against S. aureus and E. coli. Dutta J. et al., (2012)
Conclusion
The result showed that 1unit absorbance for medium and S.aureus at the same time or same hour of incubation S. aureus with chitosan recorded 0.28 and chitin with S.aureus found 0.64. So chitosan is about 2.2 times more active against S.aureus than chitin. While chitosan is about 3.0 times more active against E.coli than chitin. In case of E.coli 1 unit absorbance for media and E.coli at the same time or same hour of incubation with chitosan found 0.26 and chitin with E.coli was 0.78. Chemically prepared chitosan from shrimp shell is highly active antibacterial agent that native chitin. There was a contradict among researcher that chitosan is more active against gram positive or gram negative bacteria. The present research demonstrate chitosan was more active against gram negative bacteria than gram positive bacteria. Chitosan containing E.coli medium absorbance was 0.02 times less than chitosan containing S. aureus.
Koide S.S., (1998) stated that chitin and chitosan in vitro showed antibacterial and anti-yeast activities. One of chitosan derivatives, i.e., N-carboxybutyl chitosan, was tested against 298 cultures of different pathogenic microorganisms that showed bacteriostatic and bactericidal 16 activities, and there were marked morphological alterations in treated microorganisms when examined by electron microscopy according to Muzzarelli R, et al., (1990). There was a contradict among researcher, Chen YM, Chung YC, Wang LW, Chen KT, Li SY cited that chitosan or its derivatives is more effective for gram-negative bacteria than gram-positive bacteria , other authors Jonathan Rhoades and Bob Rastall cited the activity of chitosan is followed by Gram-positive bacteria and finally Gram-negative bacteria. The result reveals that chitosan produced by chemical treatment from shrimp (Macrobrachium rosenbergii) waste was more active antibacterial agent, inhibits both gram negative and gram positive bacteria. So it is a broad spectrum antibacterial agent.
The major constituent of their cell wall is peptidoglycan and a little amount of protein. The cell wall of Gram negative bacteria on the other hand is thinner but more complex and contains various polysaccharides, proteins and lipids beside the peptidoglycan. Also, the cell wall of Gram-negative bacteria has an outer membrane which constitutes the outer surface of the wall (Black, 1996) the complex structure of gram positive bacteria is a possible cause of the less effective than gram negative strains.
Acknowledgement
The research was jointly supported by Department of Applied Nutrition and Food Technology Islamic University, Bangladesh and Agrochemical and Environmental Research Division, Institute of Food and Radiation Biology Division, Atomic Energy Research division, Savar, Dhaka.
References
Black, J. G. (1996). ‘Microbiology: Principles and Application,’ New Jersey: Prentice-Hall, Inc, 80—82.
Chen, C. S., Liau, W. Y. & Tsai, G. J. (1998). J. Food Prot., 61, p.1124-1128
Chen, M.- C., Yeh, G. H.- C. & Chiang, B.- H. (1996). “Antimicrobial and Physicochemical Properties of Methylcellulose and Chitosan Films Containing a Preservative,” J. Food Proc. Preserve. 20(5) 275-390.
Publisher – Google Scholar – British Library Direct
Chung, Y. C., Su, Y. P., Chen, C. C., Jia, G., Wang, H. L., Wu, J. C. G. & Lin, J. G. (2004). ‘Acta Pharmacol,’ Sinica, 25, p.932-936.
Coma, V., Deschamps, A. & Martial-Gros, A. (2003). “Bioactive Packaging Materials from Edible Chitosan Polymer–Antimicrobial Activity Assessment on Dairy-Related Contaminants,” J. Food Sci., 68, p.2788-2792
Publisher – Google Scholar – British Library Direct
Dutta, J., Tripathi, S. & Dutta, P. K. (2012). “Progress in Antimicrobial Activities of Chitin, Chitosan and its Oligosaccharides: A Systematic Study Needs for Food Applications,” Food Science and Technology International. 18 (1) 3-34.
Publisher – Google Scholar
Dutta, P. K., Tripath, S., Mehrotra, G. K. & Dutta, J. (2009). “Perspectives for Chitosan Based Aantimicrobial Films in Food Applications,” Food Chem, 114, p.1173-1182
Publisher – Google Scholar
Eaton, P., Fernandes, J. C., Pereira, E., Pintado, M. E. & Malcata, F. X. (2008). “Atomic Force Microscopy Study of the Antibacterial Effects of Chitosans on Escherichia Coli and Staphylococcus Aureus,” Ultramicroscopy. (108) 1128-1134.
Publisher – Google Scholar
Fernandes, J. C., Tavaria, F. K., Soares, J. C., Ramos, O. S., João Monteiro, M., Pintado, M. E. & Xavier M. F. (2008). “Antimicrobial Effects of Chitosans and Chitooligosaccharides upon Staphylococcus Aureus and Escherichia coli in Food Model Systems,” Food Microbiol. (25) 922-928.
Publisher – Google Scholar
Jayakumar, R., Nwe, N. T., Tokura, S. & Tamura, H. (2007). “Sulfated Chitin and Chitosan as Novel Biomaterial,” Int. J. Biol. Macromol. (40) 175-181.
Publisher – Google Scholar
Jeon, Y. J., Park, P. J. & Kim, S. K. (2001). Carbohyd. Polym., 44, p.71-76
Koide, S. S. (1998). “Chitin-Chitosan: Properties, Benefits and Risks,” Nutrition Research. 18(6) 1091-1101.
Publisher – Google Scholar – British Library Direct
Kumar, A. B. V., Varadaraj, M. C., Gowda, L. R. & Tharanathan, R. N. (2005). “Characterization of Chito-oligosaccharides Prepared by Chitosanolysis with the Aid of Papain and Pronase, and their Bactericidal Action against Bacillus Cereus and Escherichia Coli,” Biochem. J., 391, 167-175.
Publisher – Google Scholar – British Library Direct
Masson, M., Holappa, J., Hjálmarsdóttir, M., Rúnarsson, Ö. V., Nevalainen, T. & Järvinen, T. – Carbohyd. (2008). Polym., 74, 566-571
Muzzarelli, R., Tarsi, R., Fillippini, o., Giovanetti, E., Biagini, G. & Varaldo, P. E. (1990). ‘Antimicrobial Properties of N-Carboxylbutyl Chitosan,’ Antimicro Ag Chemotherap. (34) 2019- 2023.
No, H. K. & Meyers, S. P. (1995). “Preparation and Characterization of Chitin and Chitosan-A Review,” Journal of Aquatic Food Product Technology. 4(2). 27-52.
Publisher – Google Scholar – British Library Direct
No, H. K., Park, N. Y., Lee, S. H., Hwang, H. J. & Meyers, S. P. (2002). “Antibacterial Activities of Chitosans and Chitosan Oligomers with Different Molecular Weights on Spoilage Bacteria Isolated from Tofu,” Journal of Food Science. 67(4). 1511-1514.
Publisher – Google Scholar – British Library Direct
No, H. K., Park, N. Y., Lee, S. H. & Meyers, S. P. (2002). “Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights,” International Journal of Food Microbiology. 74. p.65-72.
Publisher – Google Scholar
Rout, S. K. (2001). ‘Physicochemical Functional and Spectroscopic Analysis of Crawfish Chitin and Chitosan as Affected by Process Modification,’ Dissertation.
Google Scholar








