Discussion
Antimicrobial stewardship is an evidence based strategy to manage the emergence of increasingly resistant organisms through prudent prescribing. The focus on delivery of such programmes has increased over the last few years as noted by Bal et al (2011), with particular emphasis on the role of broad spectrum antibiotics and de-escalation. At the heart of the delivery of these new methodologies is a seamless interface between the laboratory susceptibility data, the medical microbiologist and the clinician.
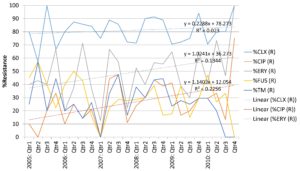
Our data has demonstrated a high level of resistance to cloxacillin in all of our CoNS isolates, with a slow increase in this level over time. Whilst this trend is not statistically significant (p>0.05) and could be attributable to statistical variability, the suggestion is that β-lactams are no longer appropriate in the empirical antimicrobial management of neonates in whom a CoNS is suspected to be significant. An increase in this resistance is in part due to the increasing use of ï¢-lactams as first and second line agents in neonatal sepsis. This would suggest a more prominent role for glycopeptides as empiric agents when clinical sepsis is suspected. The use of techniques for the standardisation of blood sampling aim to reduce contamination rates, although early cessation of therapy is recommended if the blood culture result is deemed a contaminant.Marques-Minana et al (2010) noted that the use of glycopeptides in neonates is complicated by unpredictable pharmacokinetics, a narrow therapeutic window, and confusion about appropriate dosing regimens. Often therapeutic ranges of 10-20 mg/L for vancomycin are quoted (as in our unit), but these are difficult to achieve, resulting in sub-optimal therapy. For those children with bacteraemias due to meticillin-resistant S. aureus (MRSA), Hussain et al (2011) demonstrated that lipopeptides have been successfully used guided by therapeutic drug monitoring.
Stuart et al (2011) noted that whilst CoNS are emerging as important nosocomial pathogens, large scale studies confirm a heterogenity of species and wide ranges in vancomycinsusceptibility. This has not been reflected in clinical practice where identification and quantitative susceptibility testing is not performed routinely on significant CoNS isolates. The study by Moise at al (2009) concluded that higher vancomycin minimum inhibitory concentrations (MICs) are associated with poorer outcomes for Staphylococcus aureusbacteraemiaes (SAB), but no such studies have been performed on CoNS. There has been some discussion in the UK of adopting a clinical breakpoint of vancomycin of 1 mg/L for SAB, resulting in more usage of lipopeptides and oxazolidonones. Further studies should be performed to assess this practice in significant CoNSbacteraemias.
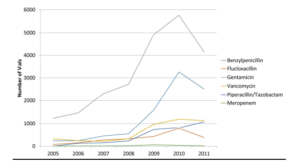
Changes in antimicrobial prescribing should be driven in part by a retrospective analysis of prescribing and consumption. The reduction in amoxicillin resistance in enterobacteraiace is driven in part by the use of aminoglycosides for the management of gram negative sepsis, which was stable practice over the study period. Our figures reflect consumption of vials on the NICU, but this cannot be standardised due to differing manufacturers and changes in administration practices over time. Our figures do suggest a large increase in consumption since 2008, the cause of which would require further investigation. The lack of WHO defined DDD’s prevents us from drawing comparisons with other centres, although Liem et al (2010) have made attempts to draw up DDDs. These have not been standardised due to the wide variation of weights in the neonatal population. It is essential for units to monitor their antimicrobial resistance rates and consumption to inform future empirical and management guidelines.
References
Bal, A. M. & Gould, I. M. (2011). “Antimicrobial Stewardship: Overcoming Implementation Barriers,” Current Opinion in Infectious Diseases, 24(4)357-62.
Publisher – Google Scholar
Hussain, A., Kairamkonda, V. & Jenkins, D. R. (2011). “Successful Treatment of Meticillin-Resistant Staphylococcus Aureus Bacteraemia in a Neonate Using Daptomycin,” Journal of Medical Microbiology, 60(Pt 3): 381-3.
Publisher – Google Scholar
Liem, T. B. Y., Heerdink, E. R., Egberts, A. C. G. & Redemaker, C. M. C. (2010). “Quantifying Antibiotic Use in Paediatrics: A Proposal for Neonatal DDDs,” European Journal of Clinical Microbiology and Infectious Diseases, 291301—1303.
Publisher – Google Scholar
Marques-Minana, M.- R., Saadeddin, A. & Peris, J.- E. (2010). “Population Pharmacokinetic Analysis of Vancomycin in Neonates. A New Proposal of Initial Dose Age Guideline,” British Journal of Clinical Pharmacology, 70 (5) 713-20.
Publisher – Google Scholar
Moise, P. A., North, D., Steenbergen, J. N. & Sakoulas, G. (2009). “Susceptibility Relationship between Vancomycin and Daptomycin in Staphylococcus Aureus: Facts and Assumptions,” Lancet Infectious Diseases, 9 (10) 617-24.
Publisher – Google Scholar
Stuart, J. I., John, M. A., Milburn, S. et al. (2011). “Susceptibility Patterns of Coagulase-Negative Staphylococci to Several Newer Antimicrobial Agents in Comparison with Vancomycin and Oxacillin,” International Journal of Antimicrobial Agents, 37 (3) 248-52.
Publisher – Google Scholar