Introduction
In 1978, Pascual-Castroviejo was the first to report coinciding anomalies (including congenital heart disease) in patients with cervicofacial hemangiomas. In view of the rarity of this combination Schneeweiss et al (1982) suggested a new syndrome of congenital cardiac and peripheral vascular anomalies. This association was further defined by Frieden et al (1996), where the acronym PHACE was introduced, later extended to PHACES with the ‘S’ reflecting supra-umbilical raphe or sternal pit. This syndrome includes Posterior fossa malformations, segmental Hemangiomas of the head and neck, Arterial anomalies, Cardiac defects, Eye anomalies and aforementioned ventral closure defects. Segmental hemangioma of the face or neck is the PHACES syndrome hallmark (Metry et al 2009, Metry et al 2009, Metry et al 2006 and Wendelin et al 2004). Cardiac involvement appears in 21 — 67% of PHACES syndrome patients [Metry et al 2009, Metry et al 2006, Haggstrom et al 2010, Metry et al 2001 and Bayer et al 2013). Aortic arch anomalies are the main cardiovascular feature, in particular coarctation of the aorta (AC), which is also associated with an increased risk of arterial ischemic stroke (AIS) [Metry et al 2009, Metry et al 2006, Metry et al 2001, Rao et al 2008, Bronzetti et al 2004, Giardini et al 2010, Siegel et al 2012 and Heyer et al 2008). In contrast to the few hundred patients reported with PHACES, infantile hemangiomas (IH) and congenital heart diseases are much more frequently diagnosed in children. The overall incidence of IH is estimated to be around 4 — 10% (Kilcline et al 2008, Jacobs 1976 and Hoornweg et al 2012), while AC has a birth prevalence of approximately 0.032%-0.036% worldwide as reported by Van der Linde et al (2011).
The pathophysiology of PHACES syndrome remains to be elucidated. A recent post-mortem study of Chad et al (2012) emphasizes the concept that an arteriopathy is the underlying defect in PHACES syndrome, resulting in dysplasia of large to medium sized arteries. From an embryologic point of view, the observation that left heart valve is absent in PHACES coarctation as mentioned by Bayer et al (2013) is supportive of this hypothesis. However, this does not directly explain the presence of hemangiomas, which are neoplastic rather than dysplastic anomalies. It is hypothesized that a delay or disturbance in the shift of embryonic to fetal vasculature might cause a hypoxic environment. Hypoxia subsequently might serve as a stimulus of IH development (Metry et al 2009 and Leaute-Labreze 2011). Current research focuses on a genetic basis for the vascular anomalies in PHACES patients. It is most likely that PHACES syndrome has a multifactorial origin with both genetic and environmental causes. In the future, large databases such as the ‘PHACE registry’ (http://www.phaceregistry.com/StudyInfo.html) might be able to either definitely prove an association between the several congenital cardiovascular defects and PHACES and to eludicate its genetic or multifactorial origin.
Most studies investigating PHACES phenomenon and the association with AC focused on patients that primarily had a (segmental) hemangioma. In sight of planning surgery for cardiac pathology, awareness of PHACES syndrome and its associated features is of in importance regarding for example, cerebral perfusion in PHACES patients. Because of the paucity of information from patients primarily diagnosed with AC, we performed a retrospective study in patients with obstructive aortic arch pathology (OAAP) in order to determine the prevalence of IH for the diagnosis PHACES syndrome in patients with aortic arch obstructions.
Methods
All pediatric patients referred to a tertiary referral centre for middle, south and east of the Netherlands between 1999 and 2013 were included. Patients were referred for diagnostic procedures or interventions (i.e. heart catherization and/or surgery) because of OAAP. OAAP was defined as AC, isolated hypoplastic aortic arch or interrupted aortic arch (IAA). A questionnaire focusing on presence of an infantile hemangioma and other symptoms in line with the diagnostic criteria of PHACES syndrome was constructed (available as online supplement). This survey including informed consent forms was sent to patients (aged > 16 years) or their parents (when aged < 16 years). Non-responders were reminded by mail one month later. When still not responding, they were reminded by telephone. We decided not to send questionnaires to parents of deceased children.
If in the questionnaire a (possible) hemangioma was reported, we asked parents or patients to send a photograph of the lesion. Following consent, medical charts were reviewed, or, when necessary, other institutions were asked for more detailed information about reported symptoms. The obtained data from the survey (age at diagnosis, location of a hemangioma, growth in time), photographs and medical chart reviews were analyzed anonymously. During a multidisciplinary meeting (two dermatologists, one plastic surgeon, one pediatric cardiologist and one pediatric hematologist, all members of the “Center of Congenital Vascular Anomalies Utrecht”; CAVU team) a diagnosis of hemangioma was confirmed or rejected. In addition it was discussed whether a subject met the criteria of PHACES syndrome in accordance with the criteria described by Metry et al (2009).
This study was judged as being not interventional by the ethics committee of the University Medical Center of Utrecht and thus did not require formal approval under Dutch law. All subjects provided informed consent for medical chart review and using information for research purposes.
Results
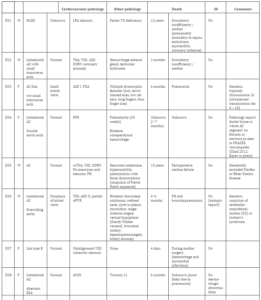
The database contained 286 children who were diagnosed with OAAP of which 90.9% had AC or an isolated hypoplastic aortic arch without intracardiac pathology. Twenty-six children (9.1%) had an IAA. Baseline patient characteristics are shown in table 1.
Table 1. Baseline Characteristics of All Patients
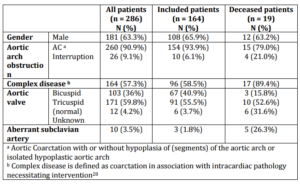
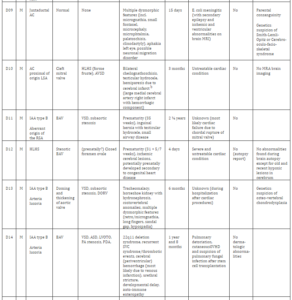
As depicted in figure 1 (flowchart), 266 questionnaires were sent, with a response rate of 66% (n = 175). Because (parental) permission to use the data for research purposes was not obtained in 11 participants, 164 (62%) surveys were eventually analyzed. Baseline characteristics were comparable for the whole group (n=286) and only those who responded to the questionnaire (i.e. “included patients”, n=164). In 23 cases (14%), patients or parents reported a possible IH. Eight of them were considered as true hemangiomas by the multidisciplinary team, based on patient history, photographs, chart review and, when available, histology reports. Characteristics of the 8 patients with a hemangioma (H01 — H08) are shown in table 2. No large (> 5cm in diameter) or segmental cervicofacial hemangiomas were diagnosed except for one patient (H09) who did not receive a questionnaire. This child was already known with PHACES syndrome, having a segmental hemangioma located mainly left sided in the periorbital and temporal region, with bilateral lesions retroauricular and a lesion of the soft palate. She had an atypical obstructed aortic arch (type C interruption) with agenesis of the left common carotid artery. During surgery a remarkable macroscopic aspect was noticed with perivascular scarring resembling like inflammatory alterations. When PHACES syndrome was suspected, further angiographic evaluation revealed hypoplasia of the left internal carotid and posterior communicating artery (major criteria) without symptoms of ischemia. In addition, the child had a sternal pit, which is also a major criterion.
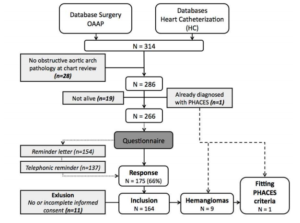
Fig. 1. Flowchart of Inclusion
Table 2. Characteristics of Hemangioma Patients
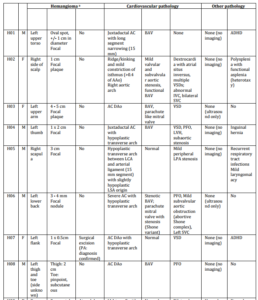
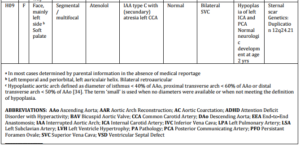
Seven other hemangioma patients had classical AC (discrete narrowing distal to the left subclavian artery (LSA)), with or without BAV (bicuspid aortic valve), not matching with the specific aortic arch anomalies in PHACES syndrome. One patient (H05) revealed a slightly atypical hypoplasia of the transverse arch with a hypoplastic origin of the LSA without other remarkable aortic arch pathology. A large or segmental hemangioma of the face, necessary for the diagnosis of PHACES syndrome, was not present in this child. No neurological or other symptoms leading to the suspicion of PHACES syndrome were reported in any of the eight participants.
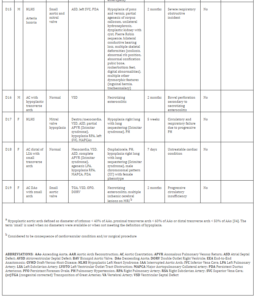
Of 286 patients with obstructive aortic arch disease, 19 patients deceased. Details of these children are summarized in table 3. None of the children had a hemangioma mentioned in their medical records or correspondence (including autopsy reports). Most children (9/19; 47%) had typical coarctation located distally of the LSA or did not have unusual characteristics of the coarctation mentioned (4/19; 21%). Two children (D10 and D15) had a coarctation proximal of the LSA and 4 children (D07, D11, D13, D14) had a type B interruption of the arch (between LCA and LSA), the most common type of IAA [20]. One child (D04) had a double aortic arch (minor criterion). None of the children had a right aortic arch. Ten patients (53%) died within the first 3 months of life, almost all due to circulatory failure as a consequence of their complex cardiac condition.
Table 3. Characteristics of Deceased Patients
Discussion
AC is a relatively common congenital abnormality. Within the PHACES spectrum, AC and IAA account for 45% of the cardiovascular anomalies as mentioned by Bayer et al (2013). As IH, the hallmark of PHACES syndrome, is highly prevalent in the healthy population, the appearance of IH in our study population of OAAP patients could also be by normal distribution. This study demonstrates that PHACES syndrome is uncommon in patients with OAAP.
IH and PHACES Prevalence
A recent prospective study among the Dutch population by Hoornweg et al (2012), showed an IH prevalence of 9.9% in newborns (0 — 16 months of age). The relatively low prevalence we found (5.5%) is most likely due to recall bias, lack of registration in medical records by physicians of these benign lesions or misdiagnoses because of the retrospective nature of assessment.
In contrast, the actual prevalence of PHACES syndrome is unknown. A frequency of 2.3% in children with IH was reported by Metry, et al (2006). In the same study, PHACES was diagnosed in 20% of IH patients with the segmental facial type (approximately 10% of all IH), which is a characteristic feature and main criterion for definite diagnosis of PHACES. Given an IH prevalence of 9.9% in the Dutch population, the prevalence of PHACES is suggested to be as high as 1:500 children. One fifth of all PHACES patients are affected by AC or IAA [10], suggesting that approximately 1 per 2500 children has PHACES with aortic arch obstruction. As the birth prevalence of AC is also around 1:2000-3000 (Van der Linde et al 2011, EUROCAT Central Registry and Website Database 2013), this would suggest that a large proportion of, if not all, AC patients could be diagnosed with PHACES.
We only found one AC patient (0.6%) with an already diagnosed PHACES syndrome in the studied cohort. This is substantially less than expected as suggested by Metry et al (2006). It should be recognized though that the cohort studied by Metry et al (2006) only concerned IH patients in tertiary dermatology centers, which may have caused selection bias that resulted in the observed high prevalence of PHACES in IH patients.
The prevalence we found here is also lower than reported by Prada et al (2010) in which 4 cervicofacial hemangiomas were shown with segmental distribution or ulceration in a cohort of 63 subjects (6.3%). We diagnosed only one child with a similar hemangioma in our cohort (0.6%). There’s no direct explanation for this remarkable difference in prevalence, but it might be partially explained by Prada et al using an outdated broad definition of Frieden et al (1996). This inclusive case definition consists of facial hemangioma plus 1 or more extracutaneous features. In contrast, we used the current diagnostic criteria as proposed by Metry et al (2009) in a consensus statement. This statement stratifies patients into 2 categories: [1] PHACES syndrome or [2] possible PHACES syndrome and uses major and minor criteria for the diagnosis.
A strong relationship between the occurrence of aortic arch obstruction and segmental IH, has been hypothesized by others (Metry et al 2009 and Bronzetti et al 2004). Despite the fact that our single PHACES case accounts for 11% of the identified hemangiomas in AC patients, which is much higher than the 2.3% of ‘general’ IH patients as reported by Metry et al (2006), our limited cohort size makes it difficult to draw definite conclusions.
Diagnostic Considerations
Except for the child with already diagnosed PHACES syndrome (H09), no patients fitted the diagnostic criteria for “definite” diagnosis of PHACES based on the hemangioma characteristics.
However, two patients with IH (H01 and H05) may be diagnosed with “possible” PHACES for having a hemangioma on the upper torso, as stated in the diagnostic criteria by Metry et al (2009). The possibility of PHACES syndrome in children with nonfacial hemangiomas is based on case reports of children with large segmental hemangiomas of the upper trunk or arm with or without minor facial involvement (Metry et al 2006 and Nabatian et al 2011). Our two ‘possible PHACES’ cases however exhibited a small (< 5 cm in diameter), non-segmental hemangioma and thus were not suspected for PHACES syndrome by our CAVU expert team. The data suggest that occurrence of IH in this cohort (except for H09) seems to be coincidental rather than related with OAAP.
The aortic abnormalities found in PHACES patients represent a distinctive morphologic entity with unusually complex and unpredictable anatomic involvement. Whereas the “classical” coarctation involves a discrete part of the proximal descending (juxtaductal) segment of the aorta, the coarctation observed in PHACES syndrome has been described to consist of a long-segment narrowing of the transverse arch with unusual dilation and aneurysm formation of adjacent arch segments (Metry et al 2006, Metry et al 2001, Rao et al 2008, Bronzetti et al 2004). These arch anomalies are often associated with abnormalities of the brachiocephalic vessels (dilation, tortuosity, and aberrant subclavian artery origin) and aortic arch sidedness as written by Metry et al (2009) and Bronzetti et al (2004).
The prevalence of BAV is as high as 50-80% in the “classic” coarctation. In PHACES syndrome however no aortic or mitral valve pathology is seen in PHACES syndrome (Metry et al 2009 and Beekman et al 2008). A recent study of 150 PHACES cases by Bayer et al (2013), showed that left heart valve pathology (BAV in particular) was completely absent in all of the 28 coarctation patients.
Our child with PHACES syndrome revealed such unusual anatomy (interruption between innominate and left common carotid artery with absence of the left carotid artery). No unusual dilation, aneurysm formation or abnormalities of the brachiocephalic vessels, in particular aberrant origin of the right subclavian artery, were observed in the other IH patients. Despite the slightly atypical aortic arch (long segment hypoplasia of arch and hypoplastic origin of LSA) in H05, this child was not considered to have PHACES. In addition, the aortic arch in the other “possible” PHACES subject (H01) did neither correspond with the bizarre arch anatomy seen in PHACES syndrome.
Deceased Patients
Another explanation for the low PHACES prevalence in our study might be that potential PHACES patients died early in life due to complications of cardio- or cerebrovascular anomalies. Therefore, we investigated the available data of deceased OAAP patients.
No hemangiomas were mentioned in medical records or autopsy reports of these children.
The diagnosis of PHACES could be deliberated in some deceased children based on certain aortic arch anomalies (e.g. double aortic arch or aberrant subclavian artery), neurologic sequelae or dysmorphic features (such as micrognathia or schizis). For all these there were arguments against this diagnosis, for example the presence of (cardiac) abnormalities which are unknown in PHACES, being diagnosed with or being suspected for another syndrome (e.g. 22q11 deletion syndrome). Unfortunately, no definite answers can be given due to lack of angiographic imaging, missing data and unreliable documentation of possible hemangiomas.
Conclusion
In this retrospective cohort of 164 OAAP patients, one child met the PHACES criteria, indicating that PHACES syndrome is rare in OAAP patients. Due to limited cohort size this study was underpowered to provide final proof of an association between IH and obstructive aortic arch pathology.
List of abbreviations
AC Aortic Coarctation
BAV Bicuspid Aortic Valve
IAA Interrupted Aortic Arch
IH Infantile Hemangioma
LSA Left Subclavian Artery
PHACES Acronym for Posterior fossa malformations, Hemangioma, Arterial anomalies, Cardiac defects, Eye anomalies, Supraumbilical raphe and/or Sternal pit
OAAP Obstructive Aortic Arch Pathology
References
Bayer, M., Frommelt, P. C., Blei, F., Breur, J. M. P. J., Cordisco, M. R., Frieden, I. J., Goddard, D. S., Holland, K. E., Krol, A. L., Maheshwari, M., Metry, D. W., Morel, K. D., North, P. E., Pope, E., Shieh, J. T., Southern, J. F., Wargon, O., Siegel, D. H. & Drolet, B. A. (2013). “Congenital Cardiac, Aortic Arch, and Vascular Bed Anomalies in PHACE Syndrome (From The International PHACE Syndrome Registry),” American Journal of Cardiology 112(12):1948-52
Publisher – Google Scholar
Beekman, R. H. (2008) [Book]. Coarctation of the Aorta, In: Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams’ Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult–Volume 2.7th Ed. Philadelphia: Lippincott Williams & Wilkins; p. 987-1004
Publisher – Google Scholar
Bronzetti, G., Giardini, A., Patrizi, A., Prandstraller, D., Donti, A., Formigari, R. et al. (2004). “Ipsilateral Hemangioma and aortic Arch Anomalies in Posterior Fossa Malformations, Hemangiomas, Arterial Anomalies, Coarctation of the Aorta, and Cardiac Defects and Eye Abnormalities (PHACE) Anomaly: Report and Review,” Pediatrics 113(2):412-5
Publisher – Google Scholar
Chad, L., Dubinski, W., Hawkins, C., Pope, E., Bernstein, S. & Chiasson, D. (2012). “Postmortem Vascular Pathology in PHACES Syndrome: A Case Report,” Pediatric and Developmental Pathology 15(6):507-10
Publisher – Google Scholar
EUROCAT Central Registry [Digital document]. Special Report: Congenital Heart Defects in Europe 2000-2005, in:Central Registry 2009. Available from: http://www.eurocat-network.eu/aboutus/publications/eurocatreportsandpapers[Accessed 2013 Jul 22]
Publisher
EUROCAT Website Database [Digital document}. EUROCAT Prevalence Data Tables; N Netherlands 2000 – 2011, in: Ulster EUROCAT Website 2013 Mar 16. Available from: http://www.eurocat-network.eu/ACCESSPREVALENCEDATA/PrevalenceTables [Accessed 2013 Jul 22]
Publisher
Frieden, I. J., Reese, V. & Cohen, D. (1996). “PHACE Syndrome. The Association of Posterior Fossa Brain Malformations, Hemangiomas, Arterial Anomalies, Coarctation of the Aorta and Cardiac Defects, and Eye Abnormalities,”Archives of Dermatology 132(3):307-11.
Publisher – Google Scholar
Giardini, A., Gholam, C., Khambadkone, S. & Kostolny, M. (2010). “Need for Comprehensive Vascular Assessment before Surgical Repair of Aortic Coarctation in PHACES Syndrome,” Pediatric Cardiology 31:291-293
Publisher – Google Scholar
Haggstrom, A. N., Garzon, M. C., Baselga, E., Chamlin, S. L., Frieden, I. J., Holland, K. et al. (2010). ”Risk for PHACE Syndrome in Infants with Large Facial Hemangiomas,” Pediatrics 126(2): e418-26
Publisher – Google Scholar
Heyer, G. L., Dowling, M. M., Licht, D. J., Tay, S. K. H., Morel, K., Garzon, M. C. & Meyers, P. (2008). “The Cerebral Vasculopathy of PHACES Syndrome,” Stroke 39(2):308-16
Publisher – Google Scholar
Hoornweg, M. J., Smeulders, M. J. C., Ubbink, D. T. & van der Horst, C. M. A. M. (2012). “The Prevalence and Risk Factors of Infantile Haemangiomas: A Case—Control Study in the Dutch Population,” Paediatric and Perinatal Epidemiology 26; 156—162
Publisher – Google Scholar
Jacobs, A. H. & Walton, R. G. (1976). “The Incidence of Birthmarks in the Neonate,” Pediatrics 58;218
Publisher – Google Scholar
Kilcline, C. & Frieden, I. J. (2008). “Infantile Hemangiomas: How Common Are They? A Systematic Review of the Medical Literature,” Pediatric Dermatology 25(2):168—173
Publisher – Google Scholar
Léauté-Labrèze, C., Prey, S. & Ezzedine, K. (2011). “Infantile Haemangioma: Part I. Pathophysiology, Epidemiology, Clinical Features, Life Cycle and Associated Structural Abnormalities,” Journal of the European Academy of Dermatology and Venereology 25(11):1245-53
Publisher – Google Scholar
Metry, D. W., Dowd, C. F., Barkovich, A. J. & Frieden, I. J. (2001). “The Many Faces of PHACE Syndrome,” The Journal of Pediatrics 139:117-123
Publisher – Google Scholar
Metry, D. W., Haggstrom, A. N., Drolet, B. A., Baselga, E., Chamlin, S., Garzon, M. et al. (2006). “A Prospective Study of PHACE Syndrome in Infantile Hemangiomas: Demographic Features, Clinical Findings, and Complications,” American Journal of Medical Genetics Part A 140A:975—986
Publisher – Google Scholar
Metry, D., Heyer, G., Hess, C., Garzon, M., Haggstrom, A., Frommelt, P. et al. for PHACE Syndrome Research Conference (2009). “Consensus Statement on Diagnostic Criteria for PHACE Syndrome,” Pediatrics 124(5):1447-56
Publisher – Google Scholar
Metry, D. W., Garzon, M. C., Drolet, B. A., Frommelt, P., Haggstrom, A. & Hall, J. (2009). “PHACE Syndrome: Current Knowledge, Future Directions,” Pediatric Dermatology 26(4):381-98.
Publisher – Google Scholar
Nabatian, A. S., Milgraum, S. S., Hess, C. P., Mancini, A. J., Krol, A. & Frieden, I. J. (2011). “PHACE without Face? Infantile Hemangiomas of the Upper Body Region with Minimal or Absent Facial Hemangiomas and Associated Structural Malformations,” Pediatric Dermatology 28(3):235-41
Publisher – Google Scholar
Pascual-Castroviejo, I. (1978). “Vascular and Nonvascular Intracranial Malformations Associated with External Capillary Hemangiomas,” Neuroradiology 16:82-84
Publisher – Google Scholar
Prada, F., Mortera, C., Bartrons, J., Rissech, M., Jiménez, L., Carretero, J. et al. (2010). “Complex Aortic Coarctation and PHACE Syndrome,” Revista Española de Cardiología 63(11):1367-70
Publisher – Google Scholar
Rao, R. P., Drolet, B. A., Holland, K. E. & Frommelt, P. C. (2008). “PHACES Association: A Vasculocutaneous Syndrome,” Pediatric Cardiology 29:793-99
Publisher – Google Scholar
Schneeweiss, A., Blieden, L. C., Shem-Tov, A., Motro, M., Feigel, A. & Neufeld, H. N. (1982). “Coarctation of the Aorta with Congenital Hemangioma of the Face and Neck and Aneurysm or Dilatation of a Subclavian or Innominate Artery. A New Syndrome?,” Chest 82(2); 186 -187
Publisher – Google Scholar
Siegel, D. H., Tefft, K. A., Kelly, T., Johnson, C., Metry, D., Burrows, P., Pope, E. et al. (2012). “Stroke in Children with Posterior Fossa Brain Malformations, Hemangiomas, Arterial Anomalies, Coarctation of the Aorta and Cardiac Defects, and Eye Abnormalities (PHACE) Syndrome: A Systematic Review of the Literature,” Stroke 43(6):1672-4
Publisher – Google Scholar
Van der Linde, D., Konings, E. E. M., Slager, M. A., Witsenburg, M., Helbing, W. A., Takkenberg, J. J. M. & Roos-Hesselink, J. W. (2011). “Birth Prevalence of Congenital Heart Disease Worldwide: A Systematic Review and Meta-Analysis,” Journal of the American College of Cardiology 58(21):2241-7
Publisher – Google Scholar
Wendelin, G., Kitzmüller, E. & Salzer-Muhar, U. (2004). “PHACES: A Neurocutaneous Syndrome with Anomalies of the Aorta and Supraaortic Vessels,” Cardiology in the Young 14:206-209
Publisher – Google Scholar










