Introduction
Imaging investigation may be used for evaluating the status of the orbital tissues. It helps to identify and differentiate Graves’ ophthalmopathy from other orbital diseases, as well (Kirsch, von Arx, 2007). Careful orbital ultrasonography and other imaging methods are used for measuring the thickness of the extraocular muscles and excluding other orbital diseases (Kirsch, von Arx, 2007; Rodriguez-Gonzalez et al, 2011; Imbrasiene et al., 2010; Nagy et al., 2000). Ultrasound scanning is the most practical and rapid non-invasive method of obtaining images of orbital tissue yielding information for diagnosis (Acharya et al., 2008).
Ultrasonography is a low cost method, without ionizing radiation. However, ultrasonography is unable to perform an accurate evaluation of the orbital apex, and the anatomy of the orbital walls. In this situation, it is better to use computed tomography and magnetic resonance imaging [Kirsch, von Arx, 2007; Kirsch et al., 2010; Dayan et Dayan, 2011).
Ultrasound findings show that Graves’ ophthalmopathy is associated with the extraocular muscle enlargement (Harris et al., 2012). Imaging methods can help to evaluate the process of inflammation and the activity of disease. (Le Moli et al., 2012; Kirsch et al., 2010; Tachibana et al., 2010).
Although thyroid diseases in children and young people are uncommon condition, therefore they can induce ocular disorders (Chan et al., 2002; Gogakos et al., 2010; Goldstein et al., 2008). Ophthalmopathy in children and young people often represents an inflammatory state of the orbital tissues (Monteiro et al., 2008).
The measurements by the usage of the thickness of extraocular muscles in juvenile patients with Graves’ disease are not widely investigated. We investigated how ultrasonography could be used for young aged patients.
The aim of our study was to evaluate the thickness of medial and inferior extraocular muscle in juvenile patients with Graves’ disease.
Materials and methods
78 orbits of 39 juvenile patients aged 6 to 34 years with Graves’ disease were investigated: 28 patients were female and 11 male.
60 orbits of 30 randomly selected normal healthy volunteers aged 7 to 33 years were evaluated in the study. 17 persons were female and 13 male.
All subjects with clinical evidence of history of thyroid, other causes of ocular protrusion (orbital inflammation, tumour, trauma), high myopia and hyperopia, and other ophthalmic disorders were excluded.
Written consent for participation was obtained from the parents of all participants or participants (in accordance to the age), and the study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of Lithuanian University of Health Sciences.
Before performing ultrasonography examination, clinical and laboratory data were recorded. The diagnosis of Graves’ disease was determined according to the clinical symptoms of Graves’ disease (diffuse goiter, hyperthyroid symptoms and signs, and increased thyroid hormone levels, suppressed serum TSH level. Ophthalmological examination included visual acuity testing (Snellen chart), biomicroscopy, ophthalmoscopy and exophthalmometry (Hertel). Orbital ultrasonography examination and extraocular muscle (medial and inferior rectus) measurement were performed with OTI-Scan ultrasonic diagnostic imaging system with a 10 MHz transducer. The probe was placed on the side of the globe opposite the muscle to be examined. All investigations were done at the Eye Clinic of the Lithuanian University of Health Sciences.
Statistical analysis
Statistical analysis was performed to describe the proptosis measurements, data of the thickness of the extraocular muscles, the mean, standard deviation (SD) using statistical software package (version 20, SPSS Inc., Chicago, IL, USA). The mean values of proptosis, extraocular muscle thickness were compared between the patients and the normal subjects (Student’s t-test). All p values were considered statistically significant when the values were
Results
Our results showed that 71.79% of the patients were female; the mean age of patients was 18.1±3.2 years. The duration of the illness was less than one year in 20 patients (51.28%), one to two years in 10 patients (25.64 %), and more than two years – in 9 patients (23.08%).
Proptosis measurements in juvenile patients with Graves’ disease ranged from 14.5 to 23.5 mm, and the mean of proptosis was 17.93± 2.3 mm (p<0.001) compared with the control group (14.07± 1.7 mm).
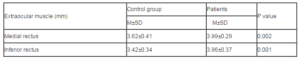
Investigations of ultrasonography showed that in healthy young persons the thickness of medial rectus muscle was 3.62±0.41 mm and of inferior rectus muscle — 3.42±0.34 mm.
The study results revealed that statistically significant enlargement was observed in medial rectus muscle (3.99±0.29 mm) and in inferior rectus muscle thickness (3.96±0.27 mm) in patients with Graves’ disease (Table 1).
Discussion
Ultrasound is a very important and accurate investigation of orbital changes in Graves’ disease and other orbit diseases (Byrne and Green, 1992; Imbrasiene, 2011; Acharya et al, 2011). Since it is non-invasive, relatively inexpensive, quick, done in real-time survey, it can be used in children and adolescents with orbital disorders. Recently, the number of children suffering from thyroid diseases is growing. Graves’ ophthalmopathy is the most frequent condition of thyroid disease. Their family doctors, pediatricians, and endocrinologists first see the majority of such patients. The determination of diagnosis is often delayed which causes the complications of this disease, such as corneal ulcerations, opacities, optic neuropathy, and myopathy. Therefore, the need for these patients to perform a nondestructive and noninvasive investigation as ultrasound examination of extraocular muscles in order to know the proptosis reason. – CT scan or nuclear magnetic resonance performing is important for differential diagnosis of orbital pathology. a
In clinical practice, ultrasonography may be used to measure the thickness of the extraocular muscles and exclude other pathology of the orbit, to differentiate the active phase from the inactive phase of Graves’ ophthalmopathy. We wanted to draw attention to the practical application of ultrasonography in the evaluation of the thickness of extraocular muscles in patients with Graves’ disease. In our study, the thickness of the medial and inferior extraocular muscles of juvenile patients with Graves’ disease was enlarged and mostly symmetrical. This fact confirms that the autoimmune process is symmetric. A-scan Ultrasonography revealed from low to medium internal reflectivity during the active inflammatory phase, and higher when the later stage of Graves’ ophthalmopathy developed.
Various studies demonstrated extraocular muscle changes in the inflammatory phase of Graves’ disease suggesting that ultrasonography is a reliable tool for the determination and observation of disease activity (Kahaly, 2001; Sabharwal et al., 2006; Gonçalves et al., 2012). In Graves’ ophthalmopathy, the inflammatory cells occur in the orbital tissues and in the surrounding extraocular muscles (Tachibana et al., 2010; Dayan et Dayan, 2007). The present study data are similar to Murakami Y et al (2001), Petrović M J et al (2012) and our studies (Imbrasiene et al., 2010). Some studies showed that the enlarged extraocular muscles could be detected by A-scan or B-scan ultrasonography or by computerized tomography and magnetic resonance imaging, as the A-scan high amplitude and signal location accurately reflect the internal structure changes of the muscles.
Ultrasonography can be a practical approach in many diseases, especially when computer tomography or magnetic resonance imaging is less available, less accessible or not (such as children, adolescents, when patient is pregnant or breast-feeding). Ultrasonography is valuable not only to monitor the early developmental stage of Graves’ disease, but also as an explanation of the confusing clinical picture and differential diagnosis, conservative and surgical treatment planning, health care and rehabilitation effects, and disease prevention.
The limitation of our research study was the small number of juvenile patients who were studied. We hope that the number of patients will increase in subsequent studies. Ultrasonic measuring of medial and lateral rectus muscles in the studies of children, adolescents and young patients with Graves’ disease are suitable for the correct diagnosis in the practice. Ultrasound investigations are very important in diagnosing Graves’ disease and may be used in evaluating the effectiveness of the treatment of this disease.
Conclusions
We measured the thickness of medial and inferior extraocular muscles in children and young people with Graves’ disease using orbital ultrasonography. The results of ultrasound investigation showed significant enlargement of medial and inferior extraocular muscle thickness in children and young patients with Graves’ disease. The data showed that ultrasonography is important in evaluating the thickness of medial and inferior extraocular muscle in Graves’ disease.
References
1. Acharya, U.R., Yun, W.L., Ng E.Y.K., Yu W. and Suri J.S. (2008) ”Imaging Systems of Human Eye: A review,” Journal of Medical Systems, 32 (4) 301-315
Publisher – Google Scholar
2. Basevicius A., Lukosevicius S., Kiudelis J., Dobrovolskiene L., Jonaitiene E. and Atkocius V. (2005) Basics of radiology. KMU, Kaunas, 128-130.
3. Byrne S.F. and Green R.L. (1992) Ultrasound of the Eye and Orbit, St. Louis: Mosby Yearbook, 215-220.
4. Kirsch E., von Arx G.and Hammer B. (2009) “Imaging in Graves’ orbitopathy,” Orbit, 28 (4) 219-225.
Publisher – Google Scholar
5. Chan W., Wong G.W.K., Fan D.S.P., Cheng A.C.K., Lam D.S.C. and Ng J.S.K. (2002) “Ophthalmopathy in childhood Graves’ disease,” British Journal of Ophthalmology, 86 (7) 740—742.
Publisher – Google Scholar
6. Dayan C.M.and Dayan M.R. (2007) “Dysthyroid optic neuropathy: a clinical diagnosis or a definable entity?” British Journal of Ophthalmology, 91 (4) 409-410.
Publisher – Google Scholar
7. Gogakos A.I., Boboridis K. and Krassas G.E. (2010) “Pediatric aspects in Graves’ orbitopathy,” Pediatric Endocrinology Reviews, 7(Suppl 2) 234-244.
8. Goldstein S.M., Katowitz W.R., Moshang T. and Katowitz J.A. (2008) “Pediatric thyroid-associated orbitopathy: the Children’s Hospital of Philadelphia experience and literature review,” Thyroid, 18(9) 997-999.
Publisher – Google Scholar
9. Gonçalves A.C., Gebrim E.M. and Monteiro M.L. (2012) “Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy,” Clinics (Sao Paulo), 67(11) 1327—1334.
Publisher – Google Scholar
10. Harris M.A., Realini T., Hogg J.P. and Sivak-Callcott J.A. (2012) “CT dimensions of the lacrimal gland in Graves orbitopathy,” Ophthalmic Plastic and Reconstructive Surgery, 28(1) 69-72.
Publisher – Google Scholar
11. Imbrasiene D. (2011) Ultrasonic investigations of the orbit. DRUKA, Klaipėda, 10-13, 67-89.
12. Imbrasiene D., Jankauskiene J. and Stanislovaitiene D. (2010) “Ultrasonic measurement of ocular rectus muscle thickness in patients with Graves’ ophthalmopathy,” Medicina, 46(7) 472-476.
13. Kahaly G.J. (2001) “Imaging in thyroid-associated orbitopathy,” European Journal of Endocrinology, 145 (2) 107-118.
Publisher – Google Scholar
14. Kirsch E.C., Kaim A.H., De Oliveira M.G.and von Arx G. (2010) “Correlation of signal intensity ratio on orbital MRI-TIRM and clinical activity score as a possible predictor of therapy response in Graves’ orbitopathy—a pilot study at 1.5 T,” Neuroradiology, 52(2) 91-97.
Publisher – Google Scholar
15. Le Moli R., Pluchino A., Muscia V., Regalbuto C., Luciani B., Squatrito S. And Vignetri G. (2012) “Graves’ orbitopathy: extraocular muscle/total orbit area ratio is positively related to the Clinical Activity Score,” European Journal of Ophthalmology, 22(3) 301-308.
16. Monteiro M.L., Goncalves A.C., Silva C.T., Moura J.P., Ribeiro C.S.and Gebrim E.M. (2008) “Diagnostic ability of Barrett’s index to detect dysthyroid optic neuropathy using multidetector computed tomography,” Clinics, 63(3) 301-306.
Publisher – Google Scholar
17. Murakami Y., Kanamoto T., Tuboi T., Maeda T. and Inoue Y. (2001) “Evaluation of extraocular muscle enlargement in dysthyroid ophthalmopathy,” Japan Journal of Ophthalmology, 45(6) 622-627.
Publisher – Google Scholar
18. Nagy E.V., Toth J., Kaldi I., Damjanovich J., Mezosi E., Lenkey A., Toth L., Szabo J., Karanyi Z. and Leovey A. (2000) “Graves’ ophthalmopathy: eye muscle involvement in patients with diplopia,” European Journal of Endocrinology, 142(6) 591-597.
Publisher – Google Scholar
19. Naik V.M., Naik M.N., Goldberg R.A., Smith T.J. and Douglas R.S. (2010) “Immunopathogenesis of thyroid eye disease: emerging paradigms,” Survey of Ophthalmology, 55(3) 215-226.
Publisher – Google Scholar
20. Petrović M.J., Sarenac T., Srećković S., Petrović M., Vulović D. and Janićijević K. (2012) “Evaluation of the patients with Grave’s ophthalmopathy after the corticosteroids treatment,” Vojnosanitetski Pregled, 69(3) 249-252.
Publisher – Google Scholar
21. Rodriguez-Gonzalez N., Perez-Rico C., Lopez-Para Gimenez R., Arevalo-Serrano J., Del Amo Garcia B., Calzada Domingo L., Flores Ruiz L. and Blanco R. (2011) “Short-tau inversion-recovery (STIR) sequence magnetic resonance imaging evaluation of orbital structures in Graves’ orbitopathy,” Archives of the Spanish Society Oftalmology, 86(11) 351-357.
Publisher – Google Scholar
22. Sabharwal K.K., Chouhan A.L.and Jain S. (2006) “CT evaluation of proptosis,” Indian Journal of Radiology and Imaging, 16 (4) 683-688.
Publisher – Google Scholar
23. Tachibana S., Murakami T., Noguchi H., Noguchi Y., Nakashima A., Ohyabu Y. and Noguchi S. (2010) “Orbital magnetic resonance imaging combined with clinical activity score can improve the sensitivity of detection of disease activity and prediction of response to immunosuppressive therapy for Graves’ ophthalmopathy,” Endocrine Journal, 57(10) 853-861.