Diabetes mellitus (DM) is considered as one of the main threats to human health in the 21st century and the number of people with diabetes has increased worldwide (Adeghate 2001). Diabetes mellitus is characterized by abnormally high plasma glucose concentrations and some metabolic abnormalities that are responsible for many disease complications, including damage to the blood vessels, eyes, kidneys and nervous system (Silverthorn 2003).
Atherosclerosis and coronary heart disease are the major health problem in developed and modern societies (Law 1999). Hyperlipidemia is an associated complication of diabetes mellitus (Miller et al., 2002).
The use of herbs as medicines has increased all over the world, gaining popularity and played an important role in disease treatment especially in diabetes mellitus (Lie and Crawford 2004; and Melzig and Funki 2007). Herbs contain natural substances and active phytochemicals that can promote health and alleviate illness as reducing blood sugar, high blood cholesterol concentrations, provide some protection against cancer and stimulate the immune system. The major advantages of herbal medicine seem to be their efficacy, low incidence of side effects (if carefully used), and low cost.
Rosmarinus officinalis L., known as rosemary, is an aromatic shrub and is native to the Mediterranean region. Its leaves are commonly used as a spice and flavoring in food processing (Saito et al., 2004). It has therapeutic applications in folk medicines such as diabetes mellitus, respiratory disorders, hepatoprotective and antitumergenic activity (Sotelo-Fleix et al., 2002; Osakabe et al., 2004; and Tavafi MH and Ahmadvand 2011).
Rosemary contains antioxidant as caffeic acid, rosmarinic acid and vitamin E. The volatile oils in rosemary also help reduce inflammation that contributes to liver and heart disease (Aruoma et al., 1992).
In most studies they use the extract but here in this study we used dried rosemary leaves powder which is common in normal kitchens not the extract.
The present research was designed to evaluate the effects of dried rosemary leaves powder on blood sugar and lipid profile of streptozotocin-induced diabetic rats. The results indicate that dried rosemary leaves powder exhibits the protective effect and prove its potentials as an anti-diabetic agent.
Materials and Methods
This study was approved by the high society of scientific ethic committee of NNI (National Nutrition Institute) & GOTHI (General Organization for Teaching Hospitals and Institutes).
One hundred (100) male Sprague Dawly rats aged 3 months, weighing 184±11 gm were used in this study. All rats were housed individually in wire meshed cages. The animals were fed on a standard rat diet for 10 days for acclimatization and water was ad libitum. Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (STZ, Sigma, St. Louis, Missouri, USA) at a dose of 50 mg/ kg body weight. STZ was dissolved immediately before use in 0.05 mol/ L sodium citrate (pH 4.5). STZ-injected animals exhibited massive glycosuria and hyperglycemia within 2-3 days.
Blood was drawn from the tail vein and fasting blood glucose was measured using Bionime, Rightest, GM 300). Rats were considered diabetic only if their fasting blood glucose levels exceeded 250 mg /dl (Cetto et al., 2000). Rat diet and body weights were also recorded on a weekly basis.
The standard rat chow diet (AIN-93 M diet formulated for adult rats) was prepared according to the National Research Council (NRC) (1978) and Reeves et al., (1993).
Experimental design
Rats were divided into five groups as follows:
1. Group 1: Control rats received standard normal diet.
2. Group 2: STZ-induced Diabetic rats (Diabetes was induced by a single intraperitoneal injection of streptozotocin, 50 mg/kg body weight).
3. Group 3: STZ-induced Diabetic rats treated with glibenclamide (600 ïg/kg body weight in aqueous solution).
4. Group 4: STZ-induced Diabetic rats treated with dried rosemary leaves powder (5 gm/ 100 gm diet).
5. Group 5: Control rats treated with dried rosemary leaves powder (5 gm/ 100 gm diet).
No detectable irritation or restlessness, no noticeable adverse effect (i.e., respiratory distress, abnormal locomotion or catalepsy) was observed in STZ-induced diabetic rats treated with dried rosemary leaves powder.
Experimental procedure
At the end of the experiments (ï‚»6 weeks, 45 days), rats were fasted overnight, and then sacrificed, anesthetized under diethyl ether. Fasting blood samples were drawn and collected in tubes (plain and coated with anticoagulant). Plain tube and one coated tube with anticoagulant tube were centrifuged for separation of serum and plasma, were stored at —70 ï‚°C for determination of the following biochemical measurements; Glucose, total cholesterol, HDL-C, LDL-C, VLDL-C and triacylglycerol were estimated. HbA1C was determined in whole blood. Atherogenic index (AI) was calculated.
Assay of Biochemical Parameters
Glucose was determined using Randox kit (Barham and Trender 1972). HbA1C was determined in using Stanbio kits procedure No. 0350 (Stanbio laboratory, Boerne, Texas; DN: RBR.0350CE.00) according to Trivelli et al., (1971). Total cholesterol, TC, was determined using Bio Mérieux kit (Allain et al., 1974). Total triacylglycerol, TG, was determined using Bicon kit (Bucolo and David 1973; and Carr et al., 1993). Serum HDL-C was determined using Bio Mérieux kit (Burstein et al., 1970; and Lopes Virella et al., 1977). Serum LDL-C was determined using Bio Mérieux kit (Levy et al., 1981). VLDL-C was determined by using the following equation: VLDL-C=total cholesterol- (HDL-C+LDL-C). Atherogenic Index (AI) was calculated according to Lee and Niemann (1996) using following equation: AI= (Total cholesterol-HDL-C)/HDL-C.
Statistical analysis
The statistical significance of the data was calculated using the Student’s t-test. Data were expressed as means ± SEM for control and experimental animals. The data were analyzed using one way analysis of variance (ANOVA) followed by post hock Duncan’s test using SPSS v 11 (statistical package for social sciences). The results were considered statistically significant if the P< 0.05.
Results
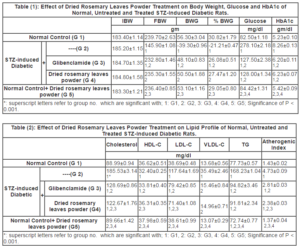
The body weight of control and experimental groups of rats were represented in Table (1). The body weight was decreased significantly in STZ-induced diabetic group of rats (-39.13%) when compared to normal control rats. Body weight was significantly increased in STZ-induced diabetic rats when treated with dried rosemary leaves powder when compared with STZ-induced diabetic group of rats.
Diabetes induced by STZ resulted in a significant elevation in the levels of fasting blood glucose (FBG) and glycosylated haemoglobin (HbA1c) in comparison to the normal control group. After treatment of diabetic group with dried rosemary leaves powder, a significant reduction (P<0.001) in FBG and HbA1c level was noted in respect to untreated (STZ) diabetic group. The treatment reduced the elevated blood glucose and HbA1c by 53.97 & 24.56% respectively in respect to untreated STZ-induced diabetic group (Table 1).
Plasma levels of TG and TC were increased significantly (P< 0.001) in STZ-induced diabetic control group when compared with control. After treatment with dried rosemary leaves powder, a significant (P< 0.001) increase was noticed in comparison with diabetic group. Regarding the levels of TG and TC in plasma, dried rosemary leaves powder treated diabetic group showed 45.43 & 33.89 % decrease in respect to STZ-induced diabetic control respectively.
Plasma levels of LDL-C and atherogenic Index (AI) was increased significantly (P< 0.001) in untreated STZ-induced diabetic group in respect to control (204.07%, 229.75 % respectively; P< 0.001). But after treatment of STZ-induced diabetic rats with dried rosemary leaves powder, the level of this biomarker was significantly decreased (P< 0.001) in respect to untreated STZ-induced diabetic group.
HDL-C level was decreased significantly (-11.53%, P< 0.001) in diabetic control group in respect to the control. But after treatment with dried rosemary leaves powder, a significant increase (P< 0.005) was noted in respect to diabetic control. The increase in plasma level of HDL-C was 12.05% in treated STZ-induced diabetic group with dried rosemary leaves powder in comparison with STZ-induced diabetic group (Table 2).
Dried rosemary powder leaves have no significant influence on body weight, plasma glucose level and lipid profile of normal rats.
Discussion
The mechanisms by which Streptozotocin brings about its diabetic state include selective destruction of pancreatic insulin secreting β-cells, which make cells less active and lead to poor glucose utilization by tissues (Lenzen, 2008).
STZ-induced diabetic rats showed a significant decrease in body weight which may be due to increased muscle wasting and loss of tissue proteins (Tavafi and Ahmadvand 2011). The improvements of body weight in STZ-induced diabetic rats treated with dried rosemary leaves powder compared with normal rats may be due to the increase of glucose metabolism, or may be due to its protective effect in controlling muscle wasting (that is the reversal of gluconeogenesis and glycogenolysis), and may also be due to the improvement in insulin secretion and glycemic control or may be due to the activation of the β-cells and granulation returned to normal, like insulinogenic effect (Chauhan et al., 2008). Rosemary stimulates insulin secretion from the remnant β-cells or regenerated β-cells and this may be through increasing betatrophin hormone (a new hormone, found in the liver that spurs the growth of insulin-secreting cells in the pancreas) secretion which increases the number of insulin-producing cells in the pancreas (Lickert 2013; and Yi et al., 2013).
The result of this study reveal that treatment of STZ-induced diabetic rats with dried rosemary leaves powder significantly reduced blood glucose level to normal glycemic level, and this trigger liver to revert to its normal homeostasis during experimental (STZ-induced) diabetes. The anti-hyperglycemic activity of dried rosemary leaves powder may be through a stimulatory effect on insulin secretion or through improvement of insulin action. Also dried rosemary may have extra pancreatic mechanism of action (Sharma et al., 2006; and Tavafi and Ahmadvand 2011) or through rosmarinic acid (one of the active dried rosemary phytochemical), which improve pancreatic β-cell function and thus enhance insulin secretion (Yeh et al., 2003). Optimal pancreatic β-cell function is essential for the regulation of glucose homeostasis in both humans and animals and its impairment leads to the development of diabetes (Fatehi-Hassanabad and Chan 2005).
Tsai et al., (2005) and Bakırel et al., (2008) attributed the anti-diabetic effect of many Labiatae species including rosemary, to their essential oil which is composed of mono sesquiterpenes; phenolic compounds and flavonoides such as caffeic acid and rosmarinic acid or to their phenolic acid content (2-3% Rosmarinic, chlorogenic, and caffeic), which had hypoglycemic effect (Al-Sereiti et al., 1999).
Glycemic control manifested by serum glucose and HbA1c was better in treated STZ-induced diabetic groups compared to untreated STZ-induced diabetics, which may suggest either sparing of more panctreatic islet cells with treatment, enhanced insulin sensitivity or insulin —like action of the dried rosemary leaves powder. The significant decrease of HbA1c in treated STZ-induced diabetic groups can be attributed to amelioration of hyperglycemia as well as the free radical scavenging activity of active components of the dried rosemary leaves powder (Mandal et al., 2008).
The result of this study reveals a significant change in plasma Lipid profile of STZ-induced diabetic rats, which appears to be a vital factor in the development of atherosclerosis which is noted in diabetes (Chattopadhyay and Bandyopadhyay 2005). Elevated levels of plasma TG and TC in STZ-induced diabetes are in agreement with Maiti et al., (2005) and Yadav et al., (2008). The present result of STZ-induced diabetic rats treated with dried rosemary leaves powder caused significant decrease in the plasma levels of cholesterol, LDL-C, VLDL-C, TG and an increase in HDL-C. This may be an indication of progressive metabolic control of dried rosemary leaf powder on mechanisms involved in the elimination of the lipids from the body. The decrease may be due to antioxidant effect of caffeic acid and its derivatives such as rosmarinic acid, which are among phytochemical constituents of rosemary leaves (Al-Sereiti et al., 1999) or because dried rosemary leaf powder might change the rate of fatty acids oxidation in the liver and reduced the rate of triglycerides biosynthesis in rats. Also rosmarinic acid or carnosic acid (derived from rosemary) inhibited LDL oxidation in a dose-dependent manner as reported by Fuhrman et al (2000). Also the decrease may be due to regeneration of the β-cells of the pancreas and potentiation of insulin secretion from surviving β-cells by dried rosemary leaves powder. The increase in insulin secretion and consequent decrease in blood glucose level may lead to inhibition of lipid peroxidation and control of lipolytic hormones (Iweala and Oludare 2011). HDL has cardio protective properties including the ability to protect LDL from oxidative modification (Nofer et al., 2002). Rosemary has high flavonoids contents, high antioxidant effect, and according to Linda et al (2006), they reported that the antioxidant properties of rosemary are of particular interest in view of the impact of oxidative modification of LDL-C in the development of atherosclerosis.
Rosemary contains flavonoids, phenols, volatile oils, carnosol and carnosic acid and terpenoids (Newall 1996). Zeng and Wang 2001 reported that carnosol, carnosic acid, rosmanol and epirosmanol phenolic diterpenes of rosemary inhibit lipid peroxidation due to its antioxidant activity (Al-Sereiti et al., 1999). Rosemary is able to stabilize free radicals through donation of electrons to them. High scavenging capacity, mostly for free radicals, of rosemary is considered as one of antioxidant action mechanism (Moreno et al., 2006).
According to Ahmadvand et al., 2012 the protection of LDL by rosmarinic acid (RA: one of the active ingredients of rosemary leaves) could be due to radical scavenging capacity of various radical species, interaction with peroxyl radicals at the LDL surface, partitioning into the LDL particle, terminating chain-reactions of lipid peroxidation by scavenging lipid radicals and regenerating endogenous α-tocopherol back to its active antioxidative form and inhibiting the formation of conjugated dienes and TBARS and increasing lag time.
Conclusion
In most studies they use the extract but here in this study we used dried rosemary leaves powder which is common in normal kitchens not the extract. The results of this experiment may indicate that the dried rosemary leaves powder has a beneficial effect as an anti-diabetic agent and its complications as well as improving lipid metabolism in diabetics with no effect on normal rats.
References
1. Adeghate E (2001). Diabetes mellitus multifactorial in aetiology and global in prevalence. Arch Physiol Biochem., 109: 197-199.
Publisher
2. Ahmadvand H, Khosrobeigi A, Nemati L, Boshtam M, Jafari N, Hosseini RH and Pournia Y (2012). Rosmarinic Acid Prevents the Oxidation of Low Density Lipoprotein (LDL) In vitro. J Biol Sci., 12(5): 301-307.
Publisher – Google Scholar
3. Allain CC, Poor LS, Chan CSG, Richmond W, and Fu PC (1974). Enzymatic determination of total serum cholesterol. Clin. Chem.; 20: 470-475.
4. Aruoma,OI, Halliwell B, Aeschbach R and Loligers J (1992). Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica, 22:257-268.
Publisher – Google Scholar
5. Al-Sereiti MR, Abu-Amer KM and Sen P (1999). Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol., 37: 124-130.
6. Bakirel T, Bakirel U, Keles OU, Ulgen SG and Yardibi H (2008). In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol., 116: 64-73.
Publisher
7. Barham D and Trinder P (1972). An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst, 97: 142-145.
Publisher
8. Bucolo G, and David H (1974). Quantitative determination of triglycerides by the use of enzymes. Clin chem., 19: 476-482.
9. Burstein M, Scholnick HR, and Monfin R (1970). Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res., 11: 585-595.
10. Carr T, Andresson CJ, and Rudel LL (1993). Enzymatic determination of triglycerides, free cholesterol and total cholesterol in tissue lipid extracts. Clin. Chem.; 26: 39-42.
11. Cetto AA, Weidonfeld H, Revilla MC, and Sergio IA (2000). Hypoglycaemic effect of Equisetum mriochaetum aerial parts on STZ-diabetic rats. J. Ethnopharmacol.; 72: 129-133.
Publisher – Google Scholar
12. Chattopadhyay RR and Bandyopadhyay M (2005). Effect of Azadirachta Indica on serum lipid profile changes in normal and Streptozotocin induced diabetic rats. Afr J Biomed Res., 8: 101-104.
13. Chauhan S, Nath N and Tule V (2008). Antidiabetic and antioxidant effects of Picrorhiza kurrooa rhizome extracts in diabetic rats. Indian J Clin Biochem., 23(3): 238-242.
Publisher – Google Scholar
14. Fatehi-Hassanabad Z and Chan CB (2005). Transcriptional regulation of lipid metabolism by fatty acids: a key determinant of pancreatic β-cell function. Nutr Metab (Lond)., 2: 1-10.
Publisher – Google Scholar
15. Fuhrman B, Volkova N, Rosenblat M and Aviram M (2000). Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal., 2: 491-506.
Publisher
16. Iweala EJ and Oludare FD (2011). Hypoglycemic effect, biochemical and histological changes of Spondias mombin linn, and Painari polyandra benth. Seeds Ethanolic extracts in alloxan induced diabetic rats. J Pharma toxic., 6(2): 101-112.
17. Law MR (1999). Lowering heart disease risk with cholesterol reduction: evidence from observational studies and clinical trials. Eur Heart J Suppl., 1: S3-S8.
18. Lee, R. and Niemann, D (1996). Nutritional Assessment 2nd ed., Mosby Missou USA.
19. Levy RI (1981). Cholesterol lipoprotein, apolipoproteins, and heart disease: Present status and future properties. Clin. Chem.; 27: 653-662.
20. Lenzen S (2008). The mechanisms of action of alloxan-and Streptozotocin-induced diabetes. Diabetologia, 51: 216-226.
Publisher – Google Scholar
21. Li MK and Crawford JM (2004). The Pathology of Cholestasis. Seminars in Liver Disease. 24(1): 21—42.
Publisher
22. Lickert H (2013). Beta-trophin fuels β-cell proliferation: first step toward regenerative therapy? Cell Metab., 18(1):5-6. doi: 10.1016/j.cmet.2013.06.006.
Publisher
23. Linda C, Hemphill L, Lynne C, David R, Michael F, Craig S, Steven R, Jennifer B, Peter M, Peter G, Virginia A and Karen E (2006). Health benefits of herbs and spices: The past, the present, the future. M J A., 185: S1-S24.
24. Lopes-Virella MF, Stone PG, Ellis S, and Coldwell JA (1977). Cholesterol determination in high density lipoprotein separated by three different methods. Clin. Chem.; 23: 882-884.
25. Maiti R, Das UK and Ghosh D (2005). Attenuation of hyperglycemia in STZ induced diabetic rats by aqueous extract of seed of Tamarindus Indica. Biol Pharm Bull., 28: 1172-1176.
Publisher – Google Scholar
26. Mandal S, Barik B, Mallick C, De D and Ghosh D (2008). Therapeutic Effect of Ferulic Acid, an Etheral Fraction of Ethanolic Extract of Seed of Syzygium cumini Against Streptozotocin-Induced Diabetes in Male Rat, Methods Find. Exp Clin., 20: 1-10.
27. Melzig, MF and Funki I (2007). Inhibitors of alpha-amylase from plants- A possibility to treat diabetes mellitus type 2 by phytotherapy? Wien Med Wochenschr., 157 (13-14): 320-324.
Publisher – Google Scholar
28. Miller CJ, Dunn EV and Hashim IB (2002). Glycemic index of 3 varieties of dates. Saudi Med J., 23(5): 536-538.
29. Moreno S, Scheyer T, Romano CS and Vojnov AA (2006). Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Research, 40 (2): 223-231.
Publisher – Google Scholar
30. National Research Council (NRC) Committee on Animal Nutrition. (1978): Nutrient requirement of laboratory animals. No. 10 3rd revised edition. National academy of science, National Research Council, Washington, DC.
31. Newall CA 1996. Herbal medicines-A guide for health care professionals. The pharmaceutical press, London, UK.
32. Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A (2002). HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis, 161: 1-16.
Publisher – Google Scholar
33. Osakabe N, Yasuda A, Natsume M and Yoshikawa T (2004). Rosmarinic acid inhibits epidermal inflammatory responses: anti-carcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis, 25: 549-557.
Publisher – Google Scholar
34. Reeves PG, Nielson FH, and Fahey GC Jr (1993). Ain 93 Purified diets for laboratory rodents: Final report of the American Institute of Nutrition and HOC Writing Committee on the Reformation of the Ain 76 A rodent diet. J Nutr., 123: 1939-1952.
35. Saito Y, Shiga A, Yoshida Y, Furuhashi T, Fujita Y and Niki E (2004). Effects of novel gaseous antioxidative system containing a rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci Biotechnol Biochem., 68: 781-786.
Publisher – Google Scholar
36. Sharma SB, Nasir A, Prabhu KM and Murthy PS (2006). Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol., 104: 367-373.
Publisher – Google Scholar
37. Silverthorn DU (2003). Fisiologia integrada. Fisiologia humana. Uma abordagem integrada. 2nd ed. Barueri (SP): Manole, 657.
38. Sotelo-Felix JI, Martinez-Fong D and Muriel De la Torre P (2002). Protective effect of carnosol on CCl4-induced acute liver damage in rats. Eur J Gastroenterol Hepatol., 14: 1001-1006.
Publisher
39. Tasi TH, Tasi PJ and Ho SC (2005). Antioxidant and anti-inflammatory activities of several commonly used spices.J Food Science, 70 (1): 93-97.
Publisher – Google Scholar
40. Tavafi MH and Ahmadvand AK (2011). Tamjidipoor A. Rosmarinic Acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. Iran J Basic Med Sci., 14 (3): 275-283.
41. Trivelli LA, Ranney HM and Lai HT (1971). Hemoglobin components in patients with diabetes mellitus. N Engl J Med., 284: 353—357.
Publisher – Google Scholar
42. Yadav JP, Saini S, Kalia AN and Dangi AS (2008). Hypoglycemic and hypolipidemic activity of Ethanolic extract of Salvadora oleoides in normal and alloxan-induced diabetic rats. Ind. J. Pharmacol., 40: 23-27.
Publisher – Google Scholar
43. Yeh GY, Eisenberg DM, Kaptchuk TJ and Phillips RS (2003). Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care, 26: 1277—1294.
Publisher – Google Scholar
44. Yi P, Park JS and Melton DA (2013). Betatrophin: a hormone that controls pancreatic β-cell proliferation. Cell, 9; 153(4):747-58. doi: 10.1016/j.cell.2013.04.008.
Publisher – Google Scholar
45. Zeng W and Wang S (2001). Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem., 49 (11): 5165-5170.
Publisher – Google Scholar