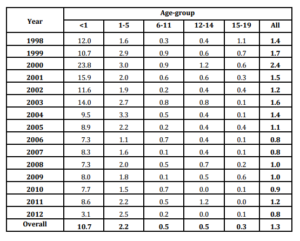
Annual incidence of all ages as one, showed a peak in 2000 (2.4 per/105 population), with smaller increases in 2003 and 2004 followed by a steady decline, nearly 1.0 per/105 population. Age-group analysis also showed the main increases from 1998 to 2000, especially in infants, but also in 2003-2004. According to gender, it was reported 359 cases among male (63.1%) and 210 cases in female (36.9%) (Data not shown).
The uppermost annual rates were observed in infants, with the highest figure during 2000 (23.8 per/105 population). After that, a slow decrease was experienced, reaching an incidence of 3.1 per/105 population in 2012 (Table 1).
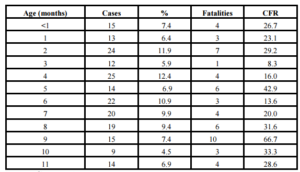
Along the period it was reported 206 cases among infants. Of them, it was possible to obtain the information about the age in months (or days) in 202 (98.0%). Main frequency of cases was observed at 4th (12.4%), 2nd (11.9%) and 6th (10.9%) months of age. Increased CFR was observed in the majority of age groups (Table 2). During neonatal period it was reported a total of 15 cases (7.4%): 4 (26.7%) patients were newborn and 3 died (CFR 75.0%); eleven patients were 7-27 days olds (73.3%) and only one died (CFR 9.1%) (Data not shown).
Table 2: Cases, percent, fatalities and case-fatality rate of pneumococcal meningitis in infants according to age in months Cuba, (1998-2012)
Case-Fatality Rate: CFR.
Monthly distribution analysis evidenced the highest numbers of cases in January, February and March (Data not shown).
Holt-Winters multiplicative analysis demonstrated the higher increases above trend of monthly average value (Root Mean Squared Error=3.63) in January (1.33%), February (1.30%) and March (1.35%), confirming the seasonality of Pnm by the dry and cold season (Figure 1).
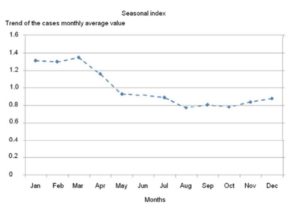 Figure 1: Seasonality of pneumococcal meningitis. Cuba, 1998-2012
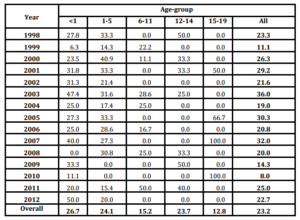
Figure 1: Seasonality of pneumococcal meningitis. Cuba, 1998-2012Table 3: Case-fatality rate of Pneumococcal Meningitis stratified according to age-group. Cuba, 1998-2012
A total of 132 fatal cases were reported during the study, for an average of 8.8 cases per year (Data not shown). Overall CFR reached 23.2% with highest figures in infants (26.7%), children 1-5 years old (24.1%) and adolescents 12-14 years old (23.7%), while the lower CFR was observed in the adolescents 15-19 years olds (12.8%). Annual CFR ranked between 36.0% (2003) and 8.0% (2010), with annual variations (Table 3).
Fatalities were more frequent among male (90) than female (42), reaching CFR of 25.1% and 20.0% respectively (Data not shown).
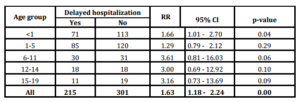
A total of 301 cases (52.9%) were admitted timely to the hospital, 215 cases (37.8%) were admitted with delay and in 53 cases (9.3%) it was not possible to obtain the information about symptoms onset date. Univariate analysis evidenced association of delayed hospitalization with the fatal outcome in age-specific groups 6-11, 12-14 and 15-19 years old with RR of 3.61 (95% CI 0.81-16.03 p>0.05), 3.00 (95% CI 0.69-12.92 p>0.05) and 3.16 (95% CI 0-73-13.69 p>0.05) respectively. When considering all age groups as one, association reach 1.63 (95% CI 0.18-2.24 p>0.05) (Table 4).
Table 4: Association of delayed hospitalization to fatal outcome. Pneumococcal meningitis. Cuba, 1998-2012
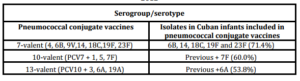
The serogroups/serotypes of S. pneumoniae identified in children and adolescents with Pnm were 6A, 6B, 7F, 9N, 14, 15C, 18C, 19A, 19F and 23F. The most common were 6B (25.8%), 6A (22.6%), 19F (12.6%) and 18C (9.7%) Non-vaccine serogroups/serotypes isolated were 9N, 15C (Data not shown). The proportions of S. pneumoniae isolates that would be strictly covered by serotypes contained within the licensed 7-valent, 10-valent and 13-valent pneumococcal conjugate vaccines were 71.4, 60.0 and 53.8% respectively (Table 5).
Table 5: Distribution of Streptococcus pneumoniae serogroups/serotypes in infants with pneumococcal meningitis covered by the pneumococcal conjugate vaccines Cuba, 2000-2012
Discussion
Results from this study need to be interpreted considering some potential limitations. As occurs in many developing and transitional countries, microbiological identification of the causative microorganism in hospitals was not always possible to achieve, owing to laboratory practices, lack of supplies and/or diagnostic methods, as well as to previous antibiotic therapy. For this reason, the true number of Pnm cases might be slightly higher than reported by surveillance.
However, our study has several important strengths. First, this report represents one of the largest, longest and deepest epidemiological studies of Pnm in children and adolescents registered in Cuba so far. Second, it was based on a nationwide surveillance which was “useful and proficient providing and synthesizing critical and multidisciplinary information, fulfilling all suitable surveillance system attributes” (Perez et al, 2010, p.4), guaranteeing reliability of data. Finally, our research was performed in a geographically well-defined population where medical care for every citizen is nationwide, with total and free access to medical assistance, vaccination, and other health requirements.
S. pneumoniae have been identified as the major and most lethal agent causing bacterial meningitis in many region of the world, mainly in infants, as reported by the World Health Organization (2003), Jeena (2006) and Zaidi et al (2009). The extensive 15-year surveillance in Cuba identified incidence rates similar to those reported in high income countries by Jeena (2006), Vergison et al (2006), Theodoridou et al (2007) and Tsai et al (2008), but lower than those declared by Mwangi et al (2002) and Zaidi et al (2009) in less-developed and transitional countries. Cases in infants during neonatal period were only a few. Beyond this period, the age distribution was analogous to those reported by Ispahani et al (2004).
However, the decrease of incidence observed in Cuba, where pneumococcal vaccination has not been implemented to date, is complex to be elucidated only by means of the available surveillance data. Similarly, Pérez et al (2010) has reported an overall decrease of CABM in Cuba, probably as a result of prior nationwide massive and high coverage vaccinations, improvements in prevention and control of this group of diseases after the implementation of the National Prevention and Control Program for Neurological Infectious Syndrome, as well as the continuous development of public health and other social benefits accessible to the population. On the other hand, it could not be totally discarded the coincidence during the analyzed period of a low incidence phase as a part of a larger cyclic variation of disease (periodical long-lasting oscillations), but the available data do not allow to fully confirm this assumption.
After the use of H. influenzae type b conjugate vaccine targeted to children under 2 years old, Dickinson et al (2001) reported a sharp decline of meningitis caused by this pathogen during 1999. Simultaneously, Pnm increased appreciably from 1999 to 2000 (much more evident in infants), with a slow decrease onward. Explanation of this spiky increase may be multifarious. Most likely, the significant reduction of Hib after conjugate vaccination may have opened a niche for pneumococci, increasing their carriage in population, and therefore the exposure and likelihood to infection and disease as Auranen et al (2010) mentioned. Unfortunately, the existing data from surveillance are insufficient to fully corroborate this hypothesis. Replacement of bacteria and serotypes after conjugate vaccination has been previously suggested and documented in other countries by Simoes et al (2004), Von Gottberg et al (2007) and Sow et al (2009).
Differences in male/female specific incidence are coincident with those reported in other studies by Theodoridouet al (2007), Rossi et al (2009) and Mosavi-Jarrahi et al (2009), and are most likely related with some sex-specific differences in physical interaction and risk behavior previously mentioned by Hussein and Shafran (2000) and Rossi et al (2009).
Ortiz et al (2006), (2008) have described that climate in Cuba is tropical and seasonally wet. The months from May to October are generally hot and rainy, and those from November to April (winter, dry season) are characterized by lower ambient temperatures and precipitation. In our study, seasonality of Pmn was identified during dry and cold period (January-March), coincidently with Sow et al (2009). Dowell et al (2003) indicated that this pattern may be associated with gathering of families due to low temperatures. On the other hand, Greenwood (2006) observed that environmental factors during dry and cold season might be more advantageous to pneumococci as a consequence of damage to the mucosal defenses increasing the ratio of infections in case patients to carriers.
Case-fatality rate observed was upper than those reported by Haddy et al (2005) and Theodoridou et al (2007) in high income countries, despite the countrywide and full access public health system existing in Cuba.
Perez et al (2010) previously mentioned that mortality may depend on some factors: care-seeking behaviour in the population, referral practices to hospitals, opportune diagnose, timely and quality of care, underlying conditions on the host and the virulence of the strains. Adequate management of Pnm certainly requires timely hospitalization and treatment. Delay in treatment may increase the risk of an unfavorable outcome as declared by Radetsky (1992). With the criteria accepted for this study, association of delayed hospitalization with fatal outcome was more evident in older children and adolescents, although no statistical significance was found, perhaps because the small number of cases in these groups. Influence of underlying conditions, as reported by Bingen et al (2008), poor and untimely recognition of symptoms (by patients or relatives), extremely virulent strains and other factors might explain these results but will require additional studies to better define.
S. pneumoniae serotype distribution may vary according to geographic region in accordance with previous results of Hausdorf, Siber and Paradiso (2001). In a research study by Gabastou et al (2008), serotypes 14, 6B, 1, 5, 19F and 18C are the most frequent reported in Latin America and the Caribbean region; all are included in 7-valent, 10-valent and 13-valent pneumococcal conjugated vaccines. Lagos et al (2008) in Chile identified serotypes 1 and 5 as the common cause of meningitis. Results in the present study reveal that serogroups/serotypes are coincident with some circulating in the region. Therefore, the use of available pneumococcal conjugated vaccines may induce a decrease of Pnm (and other clinical presentations of pneumococcal disease), preventing fatality, disability and neurological sequel particularly among children and adolescents.
In Cuba it has not been implemented vaccination against S. pneumoniae on a national scale until now, because of economical reasons. Introduction of a suitable pneumococcal conjugate vaccine in the future will reduce significantly the incidence of pneumococcal infections. At that time, the existing epidemiological and microbiological data will be essential as a baseline to compare further changes in behavior of the disease, the circulating serogroups/serotypes, as well as the impact of vaccination.
Conclusion
Meningitis caused by S. pneumoniae affects mostly the infants and is particularly lethal in Cuba. Seasonal increase occurs in dry and cold months. Delayed hospitalization contributes to increase fatality. Main S. pneumoniae serogroups/serotypes circulating in Cuban children and adolescents are included in commercial pneumococcal conjugated vaccines. We emphasize the importance of continuous nationwide surveillance to achieve more comprehensive insights into pneumococcal epidemiology as well as their contribution to the assessment of future immunization strategies.
Acknowledgments
We thank epidemiologists who were involved in the data collection and also to all our colleagues Heads of Provincial Programs for Prevention and Control of Infectious Neurological Syndrome, as well as other staff of the Infectious Neurological Syndrome Provincial Commissions for their cooperation.
References
1. Auranen, K., Mehtala, J., Tanskanen, A. and Kaltoft, M.S. (2010) “Between-strain competition in acquisition and clearance of pneumococcal carriage-epidemiologic evidence from a longitudinal study of day-care children,”American Journal of Epidemiology, 171 169—176.
2. Bingen, E., Levy, C., Varon, E., de La Rocque, F., Boucherat, M., d’Athis, P. et al. (2008) “Pneumococcal meningitis in the era of pneumococcal conjugate vaccine implementation,” European Journal of Clinical Microbiology Infectious Diseases, 27 191—199.
Publisher – Google Scholar
3. Castañeda, E., Agudelo, C.I., Regueira, M., Corso, A., Brandileone, M.C., Brandão, A.P., maldonado, a. et al. (2009) “Laboratory-based surveillance of Streptococcus pneumoniae invasive disease in children in 10 Latin American countries: SIREVA II Project Group, 2000-2005,” Pediatric Infectious Disease Journal, 28 265—270.
Publisher – Google Scholar
4. Clarke, S.C. (2006) “Control of pneumococcal disease in the United Kingdom-the start of a new era,” Journal of Medical Microbiology, 55 975—980.
Publisher – Google Scholar
5. Dickinson F, Pérez A, Galindo M, and Quintana I. (2001) “Impact of vaccination against Haemophilus influenzae type b in Cuba”, Pan American Journal of Public Health, 10(3) 169—173.
Publisher – Google Scholar
6. Dowell, S.F., Whitney, C.G., Wright, C., Rose, C.E. and Schuchat, A. (2003) “Seasonal Patterns of Invasive Pneumococcal Disease”, Emerging Infectious Diseases, 9 (5) 73—79.
7. Gabastou J.M., Agudelo C.I., de Cunto M.C., Castañeda E., Silva A.P. and Di Fabio J.L. (2008) “Characterization of invasive isolates of S. pneumoniae, H. influenzae, and N. meningitidis in Latin America and the Caribbean: SIREVA II, 2000—2005,” Pan Am J Public Health, 24(1) 1-15.
Publisher – Google Scholar
8. Gessler, P. (2010) “Invasive pneumococcal disease in children prior to implementation of the conjugate vaccine in the Zurich region, Switzerland,” Acta Pædiatrica, 99 1005—1010.
Publisher – Google Scholar
9. Greenwood, B. (2006) “Pneumococcal Meningitis Epidemics in Africa,” Clinical Infectious Diseases, 43 701—703.
Publisher – Google Scholar
10. Haddy, R.I., Perry, K., Chacko, C.E., Helton, W.B., Bowling, M.G., Looney, S.W. et al. (2005) “Comparison of incidence of invasive Streptococcus pneumoniae disease among children before and after introduction of conjugated pneumococcal vaccine,” Pediatric Infectious Disease Journal, 24 320—323.
Publisher – Google Scholar
11. Hausdorf, W.P., Siber, G. and Paradiso, P.R. (2001) “Geographical differences in invasive pneumococcal disease rates and serotypes frequency in young children,” Lancet, 357 950—952.
Publisher – Google Scholar
12. Hsu, H.E., Shutt, K.A., Moore, M.R., Beall, B.W., Bennett, N.M. and Craig, A.S. (2009) “Effect of Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis,” New England Journal of Medicine, 360 244—256.
Publisher – Google Scholar
13. Hussein, A.S. and Shafran, S.D. (2000) “Acute bacterial meningitis: a 12 year review,” Medicine, 79 360—368.
Publisher – Google Scholar
14. Ispahani, P., Slack, R.C.B., Donald, F.E., Weston, V.C. and Rutter, N. (2004) “Twenty year surveillance of invasive pneumococcal disease in Nottingham: serogroups responsible and implications for immunization,” Archives of Disease in Childhood, 89 757—762.
Publisher – Google Scholar
15. Jeena, P.M. (2006) “The burden of pneumococcal disease in children. Advances in the fight of this epidemic,”Professional Nursing Today, 10(2) 16—22.
Publisher – Google Scholar
16. Lagos, R., Muñoz, A., San Martin, O., Maldonado, A., Hormazabal, J.C., Blackwelder, W.C. et al. (2008)
“Age – and Serotype – Specific Pediatric Invasive Pneumococcal Disease: Insights from Systematic Surveillance in Santiago, Chile, 1994—2007,” Journal of Infectious Diseases, 198 1809-1817.
Publisher – Google Scholar
17. McMaster, P., McIntyre, P., Gilmour, R., Gilbert, L., Kakakios, A., Mellis, C. (2002) “The emergence of resistant pneumococcal meningitis–implications for empiric therapy,” Archive of Disease in Childhood, 87 207—211.
Publisher – Google Scholar
18. Mosavi-Jarrahi, A., Esteghamati, A., Asgari, F., Heidarnia, M., Mousavi-Jarrahi, Y. and Goya, M. (2009) “Temporal analysis of the incidence of meningitis in the Tehran metropolitan area, 1999-2005”, Population Health Metrics. [Online], [Retrieved May 13, 2010], http://www.pophealthmetrics.com/content/7/1/19/
Google Scholar
19. Mwangi, I., Berkley, J., Lowe, B., Peshu, N., Marsh, K., and Newton, C.R. (2002) “Acute bacterial meningitis in children admitted to a rural Kenyan hospital: increasing antibiotic resistance and outcome,” Pediatric Infectious Diseases Journal, 21 1042—1048.
Publisher – Google Scholar
20. Ortiz, P.L., Pérez, A.E., Rivero, A., León, N., Díaz, M., and Pérez, A. (2006) “Assessment of human health vulnerability to climate variability and change in Cuba,” Environmental Health Perspective, 114 (12) 1942—1949.
Google Scholar
21. Organización Panamericana de la Salud e Instituto Nacional de Salud Colombia. 2004. “Programa de vigilancia de los serotipos y resistencia antimicrobiana de Streptococcus pneumoniae y Haemophilus influenzae. Manual de procedimientos del proyecto SIREVA II”. [Online] 33 — 99, [Retrieved November 30, 2005], http://www.paho.org/Spanish/AD/THS/EV/LABS-manual-vigilancia-serotipos.pdf/
22. Ortíz, P.L., Pérez, A.E., Rivero, A., Pérez, A., Cangas, J.R. and Lecha, L.B. (2008) “Variability and climate change in Cuba: potential impact on the human health”. Revista Cubana de Salud Pública. [Online] 34 (1). p. 0-0, [Retrieved June 9, 2009], http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-34662008000100008&lng=es
Google Scholar
23. Pérez, A., Dickinson, F., and LLANES, R. (2010) “Invasive meningococcal disease. Cuba 1983-2006,” VacciMonitor, 19 (3) 8—12.
Google Scholar
24. Pérez, A.E., Dickinson, F.O., and Rodriguez, M. (2010) “Community acquired bacterial meningitis in Cuba: a follow up of a decade”, BMC Infectious Diseases. [Online] 10: 130. 1-9, [Retrieved December 10, 2010], http://www.biomedcentral.com/1471-2334/10/130/
Google Scholar
25. Pérez A., Dickinson f., Baly A. and Martinez R. (1999). “The Epidemiological Impact of Antimeningococcal B Vaccination in Cuba,” Mem Inst Oswaldo Cruz, 94 433-440.
Google Scholar
26. Radetsky, M. (1992) “Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis,” Pediatric Infectious Disease Journal, 11(9) 694—97.
Publisher – Google Scholar
27. Rossi, P.G., Mantovani, J., Ferroni, E., Forcina, A., Stanghellini, E., Curtale, F., et al. (2009) “Incidence of bacterial meningitis (2001-2005) in Lazio, Italy: the results of an integrated surveillance system”. BMC Infectious Diseases. [Online]. 9: 13. 1-32, [Retrieved May 14, 2010], http://www.biomedcentral.com/1471-2334/10/130/
Google Scholar
28. Simões L.L.P., Andrade A.L.S.S, Laval C.A, Oliveira R.M, Silva S.A, Martelli C.M.T. et al (2004) “Impact of Haemophilus influenzae b (Hib) vaccination on meningitis in Central Brazil,” Revista de Saude Pública, 38(5) 664—670.
29. Sorensen, U.B. (1993) “Typing of pneumococci by using 12 pooled antisera,” Journal of Clinical Microbiology 31 2097—2100.
Google Scholar
30. Sow, S.O., Tapia, M.D., Diallo, S., Keita, M.M., Sylla, M., Onwuchekwa, U., et al. (2009) “Haemophilus influenza type b conjugate vaccine introduction in Mali: impact on disease burden and serologic correlate of protection,” American Journal of Tropical Medicine and Hygiene, 80. 1033—1038.
31. Theodoridou, M.N., Vasilopoulou, V.A., Atsali, E.E., Pangalis, A.M., Mostrou, G.J., Syriopoulou, V.P. et al. (2007) “Meningitis registry of hospitalized cases in children: epidemiological patterns of acute bacterial meningitis throughout a 32-year period”, BMC Infectious Diseases. [Online]. p. 1-30. 7 (101), [Retrieved August 18, 2010], http://www.biomedcentral.com/1471-2334/7/101/
Publisher – Google Scholar
32. Tsai, C.J., Griffin, M.R., Nuorti, P. and Grijalva, C.G. (2008) “Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States,” Clinical Infectious Diseases, 46 1664—1672.
Publisher – Google Scholar
33. Vergison, A., Tuerlinckx, D., Verhaegen, J. and Malfroot, A. (2006) “Epidemiologic Features of Invasive Pneumococcal Disease in Belgian Children: Passive Surveillance Is Not Enough,” Pediatrics, 118(3) 801—809.
Publisher – Google Scholar
34. Von Gottberg, A., de Gouveia, L., Madhi, S.A., du Plessis, M., Quan, V., Soma, K., et al. (2006)
“Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa,” Bulletin of World Health Organization, 84 811—818.
Publisher – Google Scholar
35. Winther, T.N., Kristensen, T.D., Kaltoft, M.S., Konradsen, H.B., Knudsen, J.D. and Birthe, H. (2008) “Invasive pneumococcal disease in Danish children, 1996-2007, prior to the introduction of heptavalent pneumococcal conjugate vaccine,” Acta Paediatrica, 98(2) 328—331.
36. World Health Organization. (2003). “Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world: Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae, Neisseria gonorrhoeae, Salmonella serotype Typhi, Shigella, and Vibrio cholerae,” [Online], World Health Organization [Retrieved January 22, 2004], http://www.who.int/entity/csr/resources/publications/drugresist/en/IAMRmanual.pdf?ua=1
Google Scholar
37. Vaccine Assessment and Monitoring team of the Department of Vaccines and Biologicals. (2003). “WHO-recommended surveillance standards for surveillance of selected vaccine-preventable diseases,” [Online] World Health Organization [Retrieved January 13, 2009], http://www.who.int/vaccines-documents/
Google Scholar
38. World Health Organization. (2007) “Pneumococcal conjugate vaccine for childhood immunization-WHO position paper,” Weekly Epidemiological Records, 82 93—104.
39. Zaidi, A.K.M., Khan, H., Lasi, R., Mahesar, W. and the Sindh meningitis Group. (2009)
“Surveillance of Pneumococcal Meningitis among Children in Sindh, Southern Pakistan,” Clinical Infectious Diseases 48 S129—135.
Publisher – Google Scholar