Introduction
Plastination was invented in 1978 at the University of Heidelberg by Doctor Gunther von Hagens. Since its prologue, it has gained wide acceptance all over the world and the first paper describing this innovative method was published by Dr. von Hagens himself in 1979 (von Hagens G 1979). Plastination is a combination of science, technological phenomenon and artistic events in association with cultural aspects of life and death (Pashaei S 2010).
The process of plastination primarily involves replacement of water and lipids in biological tissues by curable polymer which is subsequently hardened. The procedure consists of the following steps – fixation, dehydration, forced impregnation in a vacuum and hardening. The ultimate properties of the finished specimen are determined by the class of polymer used which may consist of silicone, epoxy-silicone or epoxy resins (von Hagens G 1987).
Plastinated specimens are now considered as milestone in medical education. They have become an ideal teaching tool not only in anatomy but also in pathology, obstetrics, radiology and surgery (Saeed et al 2001, Al-Zuhair et al 1995). Plastinated specimens have several advantages over formalin preserved ones in that the former are clean, dry, odorless, durable, non-toxic, non-infectious, do not exude fumes or fluid, have superior esthetics, can act as patient educative tool, can be handled without gloves and do not require any special storage conditions or care (Latorre R. M 2007, Ravi SB 2011). Moreover studies have shown that plastinated specimens can be used for both light microscopy and ultra-structural studies following deplastination thus allowing retrospective epidemiological studies of archived pathology specimens (Grondin G et al 1994). Literature search revealed very few studies (Aufdemorte TB et al 1985, Vidya M et al 2009) concerning plastination of oral soft tissue specimens. With regards to plastination of teeth specimens we were unable to find any studies. Therefore present study aimed to perform and assess the efficacy of plastination technique in demonstration of root canal morphology of teeth and preservation of oral soft tissue specimen for museum purposes. Also, an attempt was made to evaluate dimensional changes in both hard and soft tissue specimens after each step of plastination procedure.
Materials
Materials required included extracted teeth, soft tissue specimen, formalin, nail enamel paint, disposable tea cups, red ink, hydrogen peroxide (for cleaning purpose), 5% nitric acid (for decalcification process), alcohol and acetone(both for dehydration purpose), methyl salicylate (for clearing process), xylene (for making embalming paste).
Methodology
Clearing and Plastination of Tooth Specimen:
20 freshly extracted teeth (single and multirooted) formed the hard tissue study sample. They were cleansed of blood and stains using hydrogen peroxide. All teeth were then measured under stereomicroscope for:
A) Cervico-occlusal (CO) dimension
B) Buccolingual (BL) or Labiolingual (LL) and Mesiodistal (MD) dimension of crown: maximum width of crown and at cementoenamel junction (CEJ)
C) BL and MD dimension of root at two points corresponding to thirds of root length.
An access cavity was prepared at CEJ and 10% formalin was injected to fix the pulp and immersed in the same solution for 24 hrs. (Figure 1) Nail enamel paint was applied to the teeth just to avoid undue staining of teeth by ink, red ink was injected at CEJ (methylene blue can be used as an alternative dye) and were dipped into the ink for 1 day.
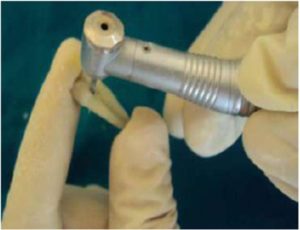
Figure 1: Showing Access Cavity Preparation at CEJ
The access preparation at CEJ was then sealed with glass ionomer cement (GIC) to prevent leaching of ink and to avoid penetration of decalcifying agent. After this, nail paint is scraped off the teeth with the bard parker (BP) blade, Teeth were then placed in 5% HNO3 (Nitric acid) for 6-8 days for decalcification, then kept under running tap water for removal of acid for 30 minutes followed by natural dehydration in sunlight till they become dry. Teeth were placed in methylsalicylate (Vatanpour M and Javidi M 2007, Omer OE et al 2004) for clearing, until they become transparent. Disposable plastic tea cups (Vidya M et al 2009) were cut into small pieces (1x1cm approximately), added to jar containing xylene, mixed properly until they form a smooth, homogenous embalming paste (Figure 2).

Figure 2: Showing Teeth Specimens Dipped in Embalming Paste
Teeth were then dipped in paste for about 24 hrs so that the paste can impregnate well then removed and allow to dry naturally for 2-3 hrs. Resulting specimen were dry and non-sticky showing dyed pulp canal morphology (Figure 3A and 3B).
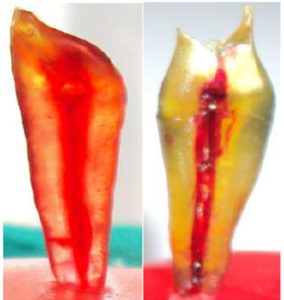
Figure 3A and 3B: Showing Teeth Specimens with Dyed Pulp Canal Morphology
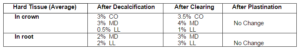
Tooth measurements were noted after decalcification, clearing and plastination process and were later compared (Table 1).
Table 1: Depicts Shrinkage of Teeth Specimen
Soft Tissue Plastination:
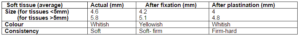
Ten formalin fixed soft tissue specimens (5 specimen of 5mm in size) were taken, followed by dehydration, firstly in 60% alcohol for 1 day followed by absolute alcohol for next 1 day after which the specimen was placed in acetone (clearing agent) for 1 hour. For plastination embalming paste was prepared similar to that used for teeth and the specimens was dipped in that for 24-36 hrs after which the specimens was removed and were allowed to dry naturally for 2-3 hours. (Vidya M et al 2009) The resulting specimens were dry, non-sticky and easy to handle. (Figure 4A and 4B) Measurement of soft tissue were done after fixation and plastination process and compared. (Table 2)
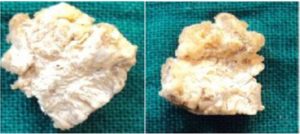
Figure 4A and 4B: Showing Plastinated Soft Tissue Specimens
Table 2: Showing Shrinkage in Soft Tissue Specimen
Results and Discussion
The dimensional changes noted for teeth were similar in single and multirooted teeth with maximum shrinkage noted in the crown portion in comparison to the root. Shrinkage was noted following both the procedures of decalcification and clearing. The procedure of plastination resulted in no appreciable change in dimension. This is in contrast to soft tissue specimen where the procedure of plastination also resulted in shrinkage. Change in colour and consistency was also noticed in soft tissue specimens.
Notwithstanding the problem of shrinkage, plastination offers several advantages for study of root canal morphology in being less expensive, offers 3D view, better contrast, less expensive and more acceptable for teaching purposes. To the best of our knowledge, this is the pioneer study utilizing plastination of cleared teeth specimens as a novel way of demonstrating root canal configuration.
We have also experienced that soft tissue plastination have numerous benefits over conventional museum techniques such as life like appearance, non- exposure to formalin, durability, stability and easy handling for teaching and maintenance free which is in accordance with von Hagens G (1987) and Latorre R. M (2007). Since in the present study we have used disposable tea cups as an alternative to conventional resin, it has tremendously reduced the cost of the technique as in the study by Vidya M et al (2009).
Conclusion
Plastination is a beautiful admixture of science and art aimed for educational purpose to both the dental students and for the patient education. Plastination in combination with clearing technique can satisfactorily demonstrate pulpal morphology of teeth. The advantage it offers makes it an ideal tool for the population based study of root canal morphology and for microleakage studies allowing three dimensional evaluations. The technique can be used adequately for conservation of routine museum specimens and also for preservation of autopsy specimens for forensic purpose.
References
Al-Zuhair, A. G. H., Butt, M. M. & Amonoo-Kuofi, H. S. (1995). ‘Plastination-A Modern Technique Supplementing the Teaching of Anatomy,’ Biomed Res, 6, 231-233.
Google Scholar
Aufdemorte, T. B., Bickley, H. C., Krauskopf, D. R. & Townsend, F. M. (1985). “An Epoxy Resin and Silicone Impregnation Technique for the Preservation of Oral Pathology Teaching Specimens,” Oral Surgery, Oral Medicine, Oral Pathology, 59(1) 74-6.
Publisher – Google Scholar
Grondin, G., Grondin, G. G. & Talbot, B. G. (1994). “A Study of Criteria Permitting the Use of Plastinated Specimens for Light and Electron Microscopy,” Biotechnic & Histochemistry, 69(4) 219-234.
Publisher – Google Scholar
Latorre, R. M., Garcia-Sanz, M. P., Moreno, M. et al. (2007). “How Useful is Plastination in Learning Anatomy?,” Journal of Veterinary Medical Education, 34, 172-6.
Publisher – Google Scholar
Omer, O. E., Al Shalabi, R. M., Jennings, M., Glennon, J. & Claffey, N. M. (2004). “A Comparison between Clearing and Radiographic Techniques in the Study of Root Canal Anatomy of Maxillary First and Second Molars,” International Endodontic Journal, 37, 291-296.
Publisher – Google Scholar
Pashaei, S. (2010). “A Brief Review on the History, Methods and Applications of Plastination,” International Journal of Morphology, 28(4), 1075—1079.
Publisher – Google Scholar
Ravi, S. B. & Bhat, V. M. (2011). “Plastination: A Novel, Innovative Teaching Adjunct in Oral Pathology,” Journal of Oral and Maxillofacial Pathology, 15, 133-137.
Publisher – Google Scholar
Saeed, M., Rufai, A. A. & Elsayed, S. E. (2001). “Mummification to Plastination,” Saudi Medical Journal, 22(11), 956—959.
Publisher – Google Scholar
Vatanpour, M. & Javidi, M. (2007). “Evaluation of Different Clearing Techniques: An Auxiliary Method for Studying Root Canal Anatomy,” The Journal of Islamic Dental Association of Iran (JIDA), 19(1), 28-33.
Publisher – Google Scholar
Vidya, M., Shetty, N., Karkera, B. V., Hemanth, M. & Shakil, M. (2009). “Plastination: An Attempt for Oral Specimen,”Indian journal of Forensic Medicine and Toxicology, 3(3), 39-40.
Publisher – Google Scholar
von Hagens, G. (1979). “Impregnation of Soft Biological Specimens with Thermosetting Resins and Elastomers,” The Anatomical Record, 194(2), 247-255.
Publisher – Google Scholar
von Hagens, G., Tiedemann, K. & Kriz, W. (1987). “The Current Potential of Plastination,” Anatomy and Embryology(Berl),175(4), 411-21.
Publisher – Google Scholar








