Introduction
Factor X is one of the vitamin K dependent factors, which is synthesized in the liver parenchymal cell and found in the plasma in a concentration of about 1 mg/dl (Mammen et al., 1983). Both inherited and acquired deficiency of Factor X is a rare bleeding disorder. Inherited deficiency of Factor X was first reported way back in mid 1950s by workers who were studying patients with haemorrhagic disease resembling factor VII deficiency. It is also known by the surnames of the patients who were first found to manifest the defect (Stuart and Prower). Congenital Factor X deficiency is inherited as an autosomal recessive condition affecting both males and females equally (Mannucci et al., 2004). It is because the complete absence of Factor X is incompatible with life, severe Factor X deficiency is one of the rarest of the known bleeding disorders (Dewerchin et al., 2000).
The exact frequency of the hereditary defect has not been established although a homozygous incidence of 1:1,000,000 has been described(Uprichard and Perry, 2002). About 50 affected families have since been reported (Roberts and Escobar, 2006). The disorder is more common in populations in which consanguineous marriage is common, such as Iran, where the frequency is reported to be 1:200 000(Karimi et al., 2002).Acquired FX deficiency occurs in up to 5% of patients with amyloidosis (Boggio and Green, 2001). There have been reports of acquired FX deficiency with cancer, myeloma, infection and use of sodium valproate. Acquired inhibitors to FX have been identified in burns, respiratory infections and exposure to topical thrombin (Dewerchin et al., 2000).
Levels Associated with Severity of Bleeding
On the basis of the plasma levels of Factor X coagulant activity (FX:C) measured with a prothrombin time-based assay using rabbit thromboplastin and Factor X-deficient plasma, patients have been classified into three groups: severe (FX:C, <1%), moderate (FX:C, 1%—5%) and mild (FX:C, 6%—10%)(Peyvandi et al., 1998). Severe clinical symptoms, such as intracranial haemorrhage (ICH), gastrointestinal bleeding and haemarthrosis, are uncommon in patients with FX:C levels >2%. In the Greifswald Factor X Deficiency Registry, the median level of FX:C in symptomatic patients was 13.3%. Patients genetically proven to be heterozygotes are usually asymptomatic but may have minor mucocutaneous bleeding symptoms. In contrast to FVIII and FIX deficiency, the most frequent bleeding symptoms are mucocutaneous: easy bruising, epistaxis and gum bleeding. Menorrhagia has been reported in 10—75% of women with severe Factor X deficiency (Razzari et al., 2006, Herrmann et al., 2006, Anwar et al., 2004). Factor X level increases in pregnancy of non-affected women (Condie, 1976), but Factor X-deficient women have been described to have uterine bleeding, fetal loss and postpartum haemorrhage.
Timing of Presentation
Factor X deficiency can present at any age and with variable manifestations .Patients with severe Factor X deficiency may present in the neonatal period with bleeding during circumcision, umbilical stump cutting, ICH or gastrointestinal haemorrhage. Moderately affected patients may be recognized only after haemostatic challenge, such as surgery, trauma or menses. Mild FX deficiency may be diagnosed during routine screening or because of a positive family history
Case Report
A 10years old girl, presented with the history of persistence of bleeding from the tooth extraction site done five days back. There was no history of bleeding from any other sites .Past history recorded appearance of bluish patches over the body even with trivial trauma and recurrent nasal bleed for last four to five years which were ignored.
There was no history of recurrent fever, progressive pallor, prolong jaundice, swelling of the body or joints, abdominal distension, any palpable lumps over the body or seizure. She had normal appetite and weight gain .There was no history of prolong intake of any drugs specially anticonvulsants, anticoagulants or antibiotics.
She was born by normal vaginal delivery at hospital with uneventful antenatal and postnatal period.
She is the first child of the family. Father denied the history of consanguinity. There was no history of similar complaints in any other member of the paternal or maternal side.
Her growth and development is normal as per her age and sex. She is good in study.
Her Immunization is completed as per national schedule.
On Examination
Her general condition was fair. There was no pallor, icterus, lymphadenopathy or edema. Echymotic patch measuring 2X3 cm2 was present over the dorsal aspect of left forearm.
Anthropometry was normal as per age and sex.
Her blood pressure was 90/60mm of Hg and pulse was 100/min with normal rhythm and good volume.
Cardiovascular, respiratory, gastrointestinal, central nervous and musculoskeletal system examinations were normal.
Examination of oral cavity showed oozing of the blood from the tooth extraction site. The child used to spit out blood mixed saliva in every 5-10minutes.
ENT examination was normal.
With above history and examination provisional diagnosis were:
1.Von willebrand disease
2.Clotting factor deficiency
3.Vitamin K deficiency
3.Thrombocytopenia
Investigations
Total and differential blood counts, platelets and ESR were within normal limit. Hemoglobin was 10.6mg%. Bleeding time was 2minutes and clotting time was 10 minutes. Both Prothrombin time (PT) and Activated partial Thromboplastin time (APTT) were prolonged. Her blood group was O positive.
Renal function, Liver function, urine and stool test including occult blood were normal. Chest X-ray , ECG and USG abdomen were normal. Tests for fibrinogen degradation product (FDP) and D-dimer were negative.
With above investigation reports provisional diagnosis were:
1.Factor I, II, V, X or XII deficiency
2.Vitamin K deficiency
3.Severe liver disease (not supporting)
4.Disseminated intravascular coagulation (DIC)( not supporting)
Other investigations helpful for proving our provisional diagnosis were sent. Till the reports came the child was managed conservatively with vitamin k injection. No improvement in APTT, PT or INR. Thus Vitamin k deficiency was ruled out. FFP and cryoprecipitate were transfused. PT decreased to 31.5( high) from 48.5 seconds with INR 2.57 from 4.03 and APTT was still 60 seconds.
Gum bleeding was tried to be control by local measures but could not. There was oozing of blood throughout the day. Her hemoglobin decreased to 8gm%. FFP and cryoprecipitate was transfused again. Packed RBC was transfused. No gum bleeding was seen thereafter.
Laboratory Data of the Patient
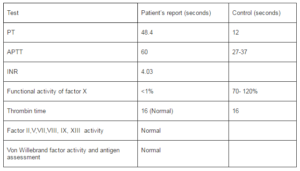
Thus based on above reports a diagnosis of severe Factor X deficiency was made.
Discussion
Factor X deficiency is an extremely rare hereditary disorder with prevalence of approximately 1 in 1 million in general population (Peyvandi et al., 1998). Affected patients may manifest with severe haemorrhagic symptoms early in life, whereas, ‘symptomatic’ heterozygote may bleed only after severe challenge to the haemostatic system, as in trauma or surgery. Bleeding sites vary according to the severity of the deficiency (Peyvandi et al., 1998). Umbilical stump bleeding may be an early manifestation of Factor X deficiency.
Soft tissue haemorrhages, including menorrhagia in women, are common in affected patients.
Haemarthrosis, exsanguinating post-operative haemorrhage, pseudotumours and central nervous haemorrhage have been reported in severely affected one.
The study done by Herrmann et al (Herrmann et al., 2006) in 35 individuals from Europe and latin America revealed the presenting symptoms as Easy bruising in (51%), Epistaxis in (34%) and Gum bleeding in (34%). Similarly study done byAnwar et al (Anwar et al., 2004) in north Pakistan showed 45%, 35% and 35% respectively of the above presenting symptoms. Our case had severe Factor X deficiency (<1%), who presented with gum bleeding, easy brusing and epistaxis at the age of nine years. Peyuandi et al (Peyvandi et al., 1998), revealed the spectrum of bleeding manifestations of 32 Iranian patients and reported that epistaxis occurred in patients with all degrees of deficiency. But other bleeding manifestations in mucosal tracts, such as haematuria and gastrointestinal bleeding occurred almost exclusively in patients with unmeasurable Factor X levels. Epistxis is unusual in patients of other coagulation factor deficiencies such as Factor VIII and IX but is prevalent in Factor X deficiency, the cause of which is not clear.
Since both PT and APTT were prolonged in our patients the activity of the clotting factors that are involved in the common (both extrinsic and intrinsic) pathway of the coagulation cascade were investigated. Except for Factor X, all came to be normal.
The acquired causes of prolonged PT and APTT could be due to specific inhibitors of Factors V, II or X, DIC, liver disease, vitamin K deficiency (malabsorption, warfarin ingestion, propylthiouracil administration), paraproteinemia and amyloidosis. Our patient did not have any of the underlying acquired causes of Factor X deficiency. Isolated Factor X concentration is not available and treatment consists of FFP which contains significant amounts of activated vitamin K — dependent factors.
References
Anwar, M., Hamdani, S. N. R., Ayyub, M. & Ali, W. (2004). “Factor X Deficiency in North Pakistan,” J Ayub Med Coll16: 1—4.
Publisher – Google Scholar
Boggio, L. & Green, D. (2001). “Recombinant Human Factor Viia in the Management of Amyloid-Associated Factor X Deficiency,” Brit J Haematol 112: 1074—5.
Publisher – Google Scholar – British Library Direct
Condie, R. G. (1976). “Serial Study of Coagulation Factors XII, XI and X in Plasma in Normal Pregnancy and in Pregnancy Complicated by Pre-Eclampsia,” Brit J OB Gyn 83: 636—9.
Publisher – Google Scholar
Dewerchin, M., Liang, Z., Moons, L., Carmeliet, P., Castellino, F. J., Collen, D. & Rosen, E. D. (2000). “Blood Coagulation Factor X Deficiency Causes Partial Embryonic Lethality and Fatal Neonatal Bleeding in Mice,”Thrombhaemost 83: 185—90.
Publisher – Google Scholar – British Library Direct
Herrmann, F. H., Auerswald, G., Ruiz-Saeza, A., Navarrete, M., Pollmann, H., Lopaciuk, S., Batorova, A. & Wulff, K. (2006). “Factor X Deficiency: Clinical Manifestation of 102 Subjects from Europe and Latin America with Mutations in the Factor 10 Gene,” Haemophilia 12: 479—89.
Publisher – Google Scholar – British Library Direct
Karimi, M., Yarmohammadi, H., Ardeshiri, R. & Yarmohammadi, H. (2002). “Inherited Coagulation Disorders in Sourthern Iran,” Haemophilia 8: 740—4.
Publisher – Google Scholar – British Library Direct
Mammen, E. F., Murano, G. & Bick, R. L. (1993). “Factor X Abnormalities,” SeminThrombHemost 9: 31-3.
Publisher – Google Scholar
Mannucci, P. M., Duga, S. & Peyvandi, F. (2004). “Recessively Inherited Coagulation Disorders,” Blood 104: 1243-52.
Publisher – Google Scholar – British Library Direct
Peyvandi, F., Mannucci, P. M., Lak, M., Abdoullahi, M., Zeinali, S., Sharifian, R. & Perry, D. (1998). “Congenital Factor X Deficiency: Spectrum of Bleeding Symptoms in 32 Iranian Patients,” Brit J Haematol 102: 626—8.
Publisher – Google Scholar – British Library Direct
Razzari, C., Martinelli, I., Bucciarelli, P., Viscardi, Y. & Biguzzi, E. (2006). “Polymorphisms of the Protein Z-Dependent Protease Inhibitor (ZPI) Gene and the Risk of Venous Thromboembolism,” Thrombhaemost 95: 909—10.
Publisher – Google Scholar – British Library Direct
Roberts, H. R. & Escobar, M. A. (2006). ‘Inherited Disorders of Prothrombin Conversion,’ In: Colman RW, Marder VJ, Clowes AW, Geroge JN, Goldhaber SZ, Eds. Hemostasis and Thrombosis — Basic Principles and Clinical Practice. 5th Edition. Philadelphia: Lippincott Williams & Wilkins, 923-37.
Uprichard, J. & Perry, D. J. (2002). “Factor X Deficiency,” Blood Rev 16: 97-110.
Publisher – Google Scholar – British Library Direct



