Introduction
The multidimensional approach to the clinical psychological and psychopathological assessment consists in the collection of data coming from different response systems like the verbal, motor, cognitive, emotional and psychophysiological one. The last one, is represented by physiological indexes such as muscular tone, heart rate, and electro dermal activity, peripheral temperature, all linked to the stress response and, more in general, to the autonomic nervous system activity. Tradition and clinical and experimental studies in psychology are filled with works and discoveries pertaining to the psychophysiological area. In particular, the theory exposed by Gray (1978) integrated by Fowle’s experimental contributes (1980; 1988) is one of the most significant, because of its applications in the diagnostic field and in the treatment evaluation. According to this theory, increase in heart rate and in electro dermal activity is the physiological manifestation of, respectively, Behavioral Activation System (B.A.S.) and Behavioral Inhibition System (B.I.S.).
The B.A.S., consisting in dopaminergic paths including the Ventral Tegmental Area (VTA) and the Nucleus Accumbens (NAcc), is activated by discriminative stimuli related to mostly positive reinforcements, but also to negative, if these ones are linked to pleasant events, and it rules the appetitive and fight or flight behavior.
B.I.S. is, instead, a network of neural structures including Amygdala, Septo-Hippocampal System, receiving serotonergic afferents from Raphe Nucleus and noradrenergic afferents from Locus Coeruleus, and projecting an efferent to the Frontal Lobe. These structures, activated by discriminative stimuli mostly related to punishment or to the reinforcement interruption, are involved in the approach-avoidance conflict. In turn, these structures, leading to the consequent behavioral arrest and to the appearance of the defensive behavior of passive avoidance.
One of the assessment techniques of the autonomic nervous response influence upon behavior is the Psychophysiological Profile (PPP), which consists in the simultaneous registration of some physiological parameters, generally divided in three continuative phases: registration at rest, stress presentation and recovery (Fuller, 1979; Hoehn, 1997).
Several physiological parameters are usually registered during a PPP, and the most used are Electromyography of the Frontal Muscle (EMG), Peripheral Temperature (PT), Heart Rate (HR), Inter Beat Interval (IBI) and Heart Rate Variability (HRV) and Skin Conductance Level (SCL). By far the most studied historically and certainly one of the most significant along with the heart rate is. While in the first (rest) and last (recovery) phase the basal autonomic activity is detected and recorded, in the halfway phase (stress), the physiological response is elicited asking to the individual to perform a mental task.
One of the most simple and used stimuli is the Mental Arithmetic Task (MAT), the continuous and serial subtraction of a two digits number (e.g. 13) from a number composed of four digits, (e.g. 1007)
Tasks of “problem solving” or of logical and analogical thought can be used for the same purpose. The Colored Progressive Matrices of Raven (CPM47) in their computerized form (Pruneti et al., 1994), in addition to maintaining a high level of attention during the whole performance, offer the opportunity to overcome any inconvenience regarding the procedures of administration and the role of the examiner (Pruneti, 1995). The principal aim of the PPP is to verify how far the psychophysiological balance seems to be maladaptive, basing on the following observations on one or more parameters:
- High level of autonomic nervous activation in the “rest” phase;
- Slow, unsettled or absent values disposition of one or more parameters during the “recovery” phase;
- Abnormal width of the activation stress-induced response in one or more parameters during the “stress” phase;
- Slow, unsettled or absent of the values of one or more parameters during the mental task (“stress” phase);
- Slow, unsettled or absent restoration of the values of one or more parameters in the “recovery” phase.
In the field of clinical psychophysiology, many studies showed the possibility to detect physiological indexes typical of some psychopathological syndromes. In fact, the presence of patterns related with the presence of anxiety and depression was first described in Lader’s researches (1975, 1983) and confirmed among others by Stegagno & Palomba (1991). While the anxious syndrome is characterized by high levels of heart rate, muscular tone, and electro dermal activity (tonic and phasic) and by a decrease in peripheral temperature, the depressive syndrome distinguishes itself by high levels of heart rate, muscular tone and low level of electro dermal activity (tonic and phasic).
Low levels of skin conductance response, at rest, were observed in depressed patients compared to a control group, (Ward & Doerr, 1986; Thorell, Kjellman & d’Elia, 1987; Argyle, 1991; Gehricke & Shapiro, 2001, Pruneti, Rota & Rossi, 2000; Pruneti et al, 2010; 2011). The meager reactivity in the same parameter in depressed individuals was verified also through imagined exposure techniques (Gehricke & Shapiro, 2001; Pruneti et al., 2010). A meta-analysis displayed that the sensitivity and specificity of electro dermal hypo reactivity for suicide in depressed patients were 97% and 93%, respectively (Thorell, 2009). There was also found that depressed patients with very high suicidal propensity had higher prevalence of hypo reactivity than those with low suicidal propensity. A recent study confirmed this finding, associating electro dermal hypo reactivity to type and severity of depression in patients with Major, Bipolar and Dystimic depression (Thorell et al., 2013).
Furthermore, in patients with panic disorder, Hoehn, McLeod & Zimmerli (1991) found a hyper activation pattern partly imposable to the one found by Lader (1975; 1983) in anxious individuals: high levels of frontal electromyography activity, systolic blood pressure and heart rate but a lower basal level in addition, a lower spontaneous electro dermal activity compared to healthy subjects. There are, however, some scientific contributes that found in patients with panic disorder high level of skin conductance level at rest (Roth et al., 1986; Roth, Ehlers, Taylor, Margraf, & Agras, 1990; Braune, Albus, Frohler, Hohn & Scheibe, 1994; Hoehn, Braune, Scheibe, & Albus, 1997; Dractu & Bond, 1998; Parente, Garcia-Leal, Del-Ben, Guimarães, & Graeff, 2005; Pruneti et al., 2010a; 2011). An elevated tonic of electro dermal activity have been also reported in literature an increased skin conductance level (SCL), reduced SCL habituation and more frequent non-specific (i.e. spontaneous) skin conductance responses (ns-SCR) (Roth et al., 1990, Braune et al., 1994 and Parente et al., 2005; Wise et al., 2011)
In patients with obsessive-compulsive disorder, a lower autonomic activation pattern was observed at rest and phasic in skin conductance level, heart rate and electromyography activity (Zahn, Leonard, Swedo, & Rapaport, 1985, Lelliott et al., 1987; Hohen, McLeod, & Hipsley, 1995; Zahn, Leonard, Swedo & Rapaport, 1996; Pruneti et al., 2011). At last, the variable of skin conductance level seems to be predictive of the possibility to decrease symptoms also in pharmacological trials (Pruneti et al., 1999; Pruneti, Giusti, Boem & Luisi, 2002; Pruneti & Luisi, 2007).
The aim of the present study is to verify through PPP and basing on the progress of skin conductance level, the autonomic activation pattern in individuals with, respectively, mostly anxious and depressive symptomatology, emphasized yet in a previous research (Pruneti et al., 2000; 2010; 2011) and in patients with obsessive-compulsive disorder and panic disorder.
Methodology and Instruments
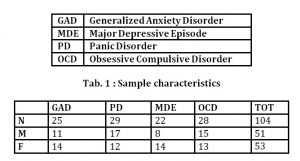
A general clinical psychological assessment was carried out on 104 patients afferent to a surgeon using, among others, the Psychophysiological Profile (PPP).
The sample, 46 males and 58 females aged between 23.4 and 51 years old (mean age= 36.4 ± 7.8), was divided into 4 groups corresponding to four different psychopathological conditions according to DSM-IV-TR criteria (American Psychiatric Association, 2000). The diagnosis had based on clinical interviews and administration of psycho diagnostic tests like Minnesota Multiphasic Personality Inventory-2 (MMPI-2; Hataway & McKinley, 1995), Symptom Questionnaire (SQ, Kellner, 1981), P Stress Questionnaire (PSQ, Pruneti, 1998; 2010).
The four diagnostic principal categories that have emerged were: Generalized Anxiety Disorder (GAD), Major Depressive Episode of average intensity (MDE), Panic Disorder (PD) and Obsessive-Compulsive Disorder (OCD) (tab.1)
All the individuals, at the moment of the PPP registration, didn’t undergo a pharmacological treatment for at least 3 months.
Inclusion-Exclusion Criteria
Individuals with any kind of organic syndromes (e.g. general medical, endocrinological, neurological, etc.) or with comorbidity with other DSM-IV Axis I and II syndromes were excluded.
As previously mentioned, the PPP was divided into the following 4 phases:
- “Adaptation” (4-5 minutes): Patients were welcomed in a room with a temperature between 18º and 22º and humidity was not higher than 50%, and they were invited to seat in an armchair with headrest, wide arms and reclining back. They were also informed about the content and the meaning of the procedure and reassured about the procedure was not invasive or harmful. At the same time, a psychologist provided for positioning electrodes and transducers, setting up the equipment for the registration and monitoring the value progress of the different physiological parameters looking for their arrangement.
- “Rest” (6 minutes): the registration started after telling the patients to close their eyes to stay still and relaxed as much as possible and to signal eventual problems ( f.e. need to cough) raising the first finger of the left hand (in that case, the registration was temporarily stopped)
- “Stress” (4 minutes): patients were asked to perform a mental task consisting in the subtraction of 13 from 1007, and from every result subsequently gained (continuous subtraction)
- “Recovery” (6 minutes): at the end of the stress, presented patients stopped performing the mental task and were invited to rest and relax as much as possible.
During the PPP the following physiological parameters were reordered:
- Electromyography of the frontal muscle (EMG), whose electric potential was detected using two active electrodes located about 1cm over the eyebrows lining with pupils, and one of reference, in the middle of the forehead.
- Heart rate (HR) and Inter Beat Interval (IBI) detecting the electric potential of the cardiac muscle with the classical bipolar junction are used for electrocardiogram and calculating the existing time between an R wave (ventricular contraction) and the other one (Heart Rate and Inter Beat Interval).
- Peripheral temperature (PT), applying a thermistor at the base of the thenar eminence of the dominant hand.
Skin Conductance Level/Response (SCL/SCR), letting pass a slight electric current between two electrodes located on the last phalanx of the dominant hand fingers (in this case, first and second finger).
All of the PPP were recorded using the Psycho-Lab VD 13 equipment of the SATEM Company in Rome, interfaced with a PC and running a specific software to collecting data and showing graphics on line.
Parameters EMG and HR were recorded trough surface disposable electrodes of 14mm.
Golden reusable electrodes detected the SCL. A precision thermistor (able to detect variation in temperature of less than1/100 °C) was utilized for PT. Signals were monitored and recorded through the software Psycholab P.C. SOFT, always belonging to the SATEM company in Rome.
Statistical Analysis
The mean values of the physiological parameters were calculated at the:
- Last minute of the “rest” phase, after the values arrangement, as a measure of the basal activation level;
- First minute of the “stress” phase, as a measure of the activation state during the performance of a mental task, in order to exclude the interference of habituation phenomena, that could lead to an underestimation of the activation width;
- Last minute of the “recovery” phase, as a measure of the activation level at the end of the procedure, in order to verify the recovery extent after a lapse of time reasonably sufficient to return to basal values.
In order to compare the groups of individuals in the three PPP phases, the following indexes were calculated:
- Stress response, as an indicator of width of the autonomic activation response, subtracting from the mean value of the first minute of the rest phase the mean value of the last minute of the recovery phase;
- After stress recovery, as an indicator of the recovery width from autonomic activation state, subtracting from the mean value of the whole stress phase (considering the mean of 4 minutes) the mean value of the last minute of the recovery phase.
In this work, only data coming from the SCL/SCR devices, will be showed and discussed. The rationale is that, as previously reported, the Galvanic Skin Response is for sure one of the most important and significant indexes of autonomic arousal.
Once verified the non-normal data distribution using the Shapiro-Wilks test, non parametric statistical analysis were employed.
A comparison between groups of subjects was carried out on the mean value of the SCL the last minute of the rest phase and on the other calculated indexes: “stress response” and “after stress recovery”. The Kruskal-Wallis non-parametric statistical test has been utilized to detect eventual differences between one or more groups and the others, and using Mann-Whitney-U-Test to verify the significance in group’s pairwise comparison.
Later, the three phases mean values were compared, separately for each group, with Friedman non-parametric statistical tests to detect eventual differences between the PPP phases, and with Wilcoxon test to evaluate the significance of the change from one phase to the other.
Significance level for the post hoc tests was determined with the Bonferroni correction according to the formula /p where p is the number of comparisons (6 in the Mann-Whitney-U-Test, and 3 in the Wilcoxon test).
Effect sizes were determined for Kruskal-Wallis with eta square (ȵ2) calculated as described by Green and Salkind (2005), for Friedman ANOVA with the Kendall’s coefficient of concordance (KW) (Siegel and Castellan, 1988), for Mann-Whitney-U-Test and Wilcoxon test with r for non-parametric statistic calculated as reported by Field (2009).
Results
From the statistical analysis, significant characteristics in the SCL recording emerged within the PPP phases and between the groups. Descriptive statistics (tab. 2, fig.1) show basal SCL values that seem to be definitely higher in GAD and PD, than in MDE and OCD.
Tab. 2 : Descriptive Statistics: mean (MA) and standard deviation (SD) of skin conductance (SCR) values registered during the last minute of rest phase , the first minute of stress phase, the last minute of recovery phase, and for the calculated indexes.
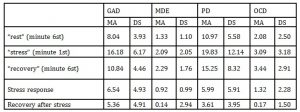
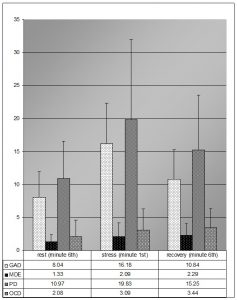
Fig. 1 : Means values and SD of skin conductance level/response in the three phases of the Psychophysiological registration (rest, stress, recovery)
The Kruskal-Wallis test verified the presence of some significant differences between the 4 psychopathological groups in the basal SCL (χ2=25.236; df=3; N=104; P<.001; ȵ2=.25) in the stress response index (χ2=17.195; df=3; N=104; P<.001; ȵ2=.17) and in the after stress recovery index (χ2=21.882; df=3; N=104; P<.001; ȵ2=.21).
In the comparison between groups, basing on the SCL values recorded at rest GAD and PD differ from MDE and OCD for a higher general level of autonomic activation. Post Hoc tests showed significant differences among either both the groups GAD or PD and the groups MDE and OCD. No significant differences were detected between GAD and PD and between MDE and OCD (tab. 3)
Tab. 3 : Skin Conductance Level/Response (SCL/SCR). Comparison between baseline and stress indexes between the four psychopathological groups: Mann-Whitney U test with Bonferroni correction and Effect Size.
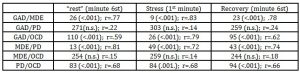
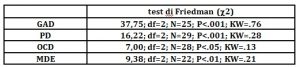
For all the four groups there were significant differences, in the Friedman test, between the PPP phases in the SCL values (tab. 4a). Post hoc comparisons showed, separately for each group, significant differences between the values of the “rest” phase and of the “stress” phase: the autonomic activation response during the mental task so occurred in all four groups (tab. 4b). Furthermore, in the comparison between groups in the stress response index, there were significant differences between the groups GAD and PD and MDE and OCD (tab.3): the width of the SCL response was higher in GAD (W= 15, P<.001; r=.79) and PD (W=16; P<.001; r=.81) groups than in MDE (W=96; P<.05; r=.46) and OCD (W=16; P<.05; r=.52) as indicated by differences in effect sizes (fig.1, tab. 4b).
Table 4a : c2 values at the Friedman’s test and effect size (Kendall’s W =KW) in the comparison of the 3 phases of the psychophysiological registration (rest, stress, recovery).
Tab. 4b: W most little value of ranks of Wilcoxon test with Bonferroni correction and Effect Size in the comparison of the different psychopathological groups.

Significant differences between the “stress” and the “recovery” phase values were found only in GAD (W=25; P<.001); r=.74) and PD (W=8; P<.001); r=.72) groups (tab. 4b): only in these two groups there was the autonomic activation response recovery.
This datum was supported from the statistic comparison between the four groups on the values of the after stress recovery index (tab. 2): the SCL response extinction is significantly higher in groups GAD and PD (fig.1).
At last, for all four groups, there were significant differences between the values of the “rest” phase and the value of the “recovery” phase [GAD, W= 41, P<.005, r=.65; PD, W=40 P<.001, r=.71; OCD, W= 91 P<.05, r=.48; MDE, W=50 P<.05, r=.53]: in all groups the reinstatement of the basal autonomic state wasn’t full-finished.
Discussion
The results of the present study verified what emphasized yet in previous works (Pruneti et al., 1999; Pruneti, 2010; Pruneti, et al. 2010; 2011). As a confirmation of some literature data: an autonomic high level of arousal and in particular a high level of SCL/SCR, appear to be typical of some of the anxious syndromes (Lader, 1975, 1983; Stegagno e Palomba, 1994). On the contrary, a low level of general autonomic arousal seems to be typical of depressive patients (Lader, 1975, 1983; Ward & Doerr, 1986; Thorell, Kjellman & d’Elia, 1987; Argyle, 1991; Gehricke & Shapiro, 2001), anorexic patients (Roth et al., 1986; Roth, Ehlers, Taylor, Margraf, & Agras, 1990; Braune, Albus, Frohler, Hohn & Scheibe, 1994; Hoehn, Braune, Scheibe, & Albus, 1997; Dractu e Bond, 1998; Parente, Garcia-Leal, Del-Ben, Guimarães, & Graeff, 2005; Pruneti et al. 2011) and Obsessive Compulsive patients (OCD) (Zahn, Leonard, Swedo, & Rapaport, 1985, Lelliott et al., 1987; Hohen, McLeod, & Hipsley, 1995; Zahn, Leonard, Swedo & Rapaport, 1996; Pruneti et al., 2010). About this syndrome (OCD), it might not be a coincidence that the last version of DSM (the 5ft, American psychiatric Association, 2013), had separate the OCD syndromes in axis I from Anxious Disorders.
Furthermore, the electro dermal activity during the PPP phases, of patients GAD, PD, and MDE confirm Gray’s B.I.S. and B.A.S. theory (1978) and Fowles’ theory (1980,1988).
The high basal level, the strong response and the not full-finished recovery of the SCL parameter should mirror the B.I.S. activation caused by internal and/or external signals of danger or personal threat. About this, Roth, Wilhem, & Trabert (1998) suggest that the meager relaxing skill of PD patients, shown by the excessive natural fluctuation and by the missed habituation of the SCL parameter, can be due to the continuous attention given to physical sensations as a part of the techniques used to control tension. Similarly, the same SCL progress in GAD patients should mirror the B.I.S. activation caused by internal stimuli like the excessive worry, also at rest, and the fear of mistakes performing the mental task. In reverse, in MDE patients the B.I.S. reduced activation, shown by the SCL low basal level and weak response, seems the psychophysiological relate of the learned helplessness condition, that is the extinction of searching for discriminative stimuli related to punishment or to reinforcements interruption, and the avoiding behavior. One more and relevant aspect of these results is their direct application in the clinical practice. Making a proper differential diagnosis in several psychopathological fields is still a complex and difficult process. Using PPP and the informations coming from SCL/SCR analysis could help, e.g. in depressive disorders to discriminate an exhaustion picture from a Major Depression Episode not only basing on the personal history and on recent life events, but also using some objective evidences. In case of a history of stressful situation, the psychophysiological data could confirm an excessive arousal that is often present in the first phase of the depressive exhaustion. A surface diagnosis could be dangerous for the patient leading to a wrong treatment plan. For example, the easy and superficial prescription of antidepressant in a depressive exhaustion syndrome could cause a hypo maniacal reaction in the subsequent two or three weeks with the risk of a more confusing diagnostic picture and with the possible prescription of different improper types of psycho drugs because the clinical picture could mimes the bipolar one.
(adsbygoogle = window.adsbygoogle || []).push({});
References
- American Psychiatric Association (APA). (2000). Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Washington DC: APA.
- Argyle, N. (1991). Skin Conductance Levels in Panic Disorders and Depression. Nervous Mental Diseases, 179 (9), 261-66.
- Braune, S., Albus, M., Frohler, M., Hohn, T. & Scheibe, G. (1994). Psychophysiological and biochemical changes in patients with panic attacks in a defined situational arousal. European Archives of Psychiatry and Clinical Neuroscience, 244(2); 86-92.
- Dractu, L.and Bond, A. (1998). Panic patients in the non-panic state: physiological and cognitive dysfunction. European Psychiatry, 13, 18-25.
- Field A. (2009) Discovering Statistics using SPSS. (3rd edition) SAGE Publications Ltd, London
- Fowles, D. C. (1980). The three-arousal model: Implications of Gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology, 17, 87-104.
- Fowles, D. C. (1988). Psychophysiology and psychopathology: A motivational approach. Psychophysiology, 25, 373-391.
- Fuller G. D., (1979). Biofeedback Methods and Procedures in Clinical Practice. S. Francisco: Biofeedback Press.
- Gehricke, J. G. and Shapiro, D. (2001). Facial and autonomic activity in depression: social context differences during imagery. International Journal of Psychophysiology, 41, 53-64.
- Gray, J. A. (1978). The neuropsychology of anxiety. British Journal of Psychology, 69, 417-438.
- Green, S. B. and Salkind, N. J. (2005). Using SPSS for Windows and Macintosh: Analyzing and understanding data (fourth edition). New Jersey:Pearson
- Hataway, S. R. and McKinley, J. C. (1995). Minnesota Multiphasic Personality Inventory – 2. Edizione italiana di S. Sirigatti e P. Pancheri. Firenze: Organizzazioni Speciali.
- Hoehn, S. R., McLeod, D. R. and Zimmerli W. D. (1991). Psychophysiological response patterns in panic disorder. Acta Psichiatrica Scandinavia, 83 (1), 4-11.
- Hoehn, T., Braune, S., Scheibe, G. and Albus, M. (1997). Physiological, biochemical and subjective parameters in anxiety patients with panic disorder during stress exposure as compared with healthy controls. European Archives of Psychiatry and Clinical Neuroscience, 247(5), 264-274.
- Hohen, S., McLeod, D. R.and Hipsley, P. (1995). Is hyperarousal essential to obsessive-compulsive disorder? Diminished physiologic flexibility, but not hyperarousal, characterizes patients with obsessive-compulsive disorder. Archives of General Psychiatry, 52 (8), 688-93.
- Insel, T. R., Zahn, T. P.and Murphy, D. L. (1985). Obsessive compulsive disorder: an anxiety disorder? In A. H. Tuma & J. Maser (eds.), Anxiety and the Anxiety Disorders (pp. 577-590) Hillside, NJ: Lawence Erlbaum Associates.
- Kellner, R. (1981). Symptom Questionnaire. Edizione italiana di G. A. Fava e R. Kellner. Firenze: Organizzazioni Speciali.
- Lader, M. H. (1975). The psychophysiology of anxious and depressed patients. In D. C. Fowles (a cura di), Clinical applications of psychophysiology. New York: Columbia University Press.
- Lader, M. H. (1983). Anxiety and depression. In A. Gale & J. A. Edwards (eds.), Physiological correlates of human behavior. Londra: Academic Press.
- Lelliott, P. T., Noshirvani, H. F., Marks, I. M., Monteiro, W. O., Basoglu, M. and Cohen, S. D. (1987). Relationship of skin conductance activity to clinical features in obsessive-compulsive ritualizers. Psychological Medicine, 17 (4), 905-14.
- Parente, A. C. B. V., Garcia-Leal, C., Del-Ben, C. M., Guimarães, F. S. and Graeff, F. G. (2005). Subjective and neurovegetative changes in healty volunteers and panic patients performing simulated public speaking. European Neuropsyhopharmacology, 15(6), 663-671.
- Pruneti, C., Giusti, M., Boem, A. & Luisi, M. (2002). Behavioral Psychophysiological and salivary cortisol after short-term alprazolam treatment in patients with recent myocardial infarction. Italian Heart Journal, 3 (1), 53-59.
- Pruneti, C., Lento, R. M., Fante, C., Carrozzo, E., Fontana, F. (2010). Autonomic Arousal and Differential Diagnosis in Clinical Psychology and Psycopathology. Italian Journal of Psychopathology, 16:43-52.
- Pruneti C., Fontana F., Fante C. (2011). Autonomic Changes and Stress response in Psychopathology. European J. of Clinical Psychology and Psychiatry.
- Pruneti, C., Fontana, F., Carrozzo, E., Fante, C. (2011). Autonomic Reactivity, Emotions and Stress Response in Psychopathology. Applied Psychophysiology and Biofeedback, 36:217-229.
- Roth, W. T., Ehlers, A., Taylor, C. B., Margraf, J. & Agras, W. S. (1990). Skin conductance habituation in panic disorder patients. Biological Psychiatry, 27 (11), 1231-43.
- Roth, W. T., Telch, M. J., Taylor, C. B., Sachitano, J. A., Gallen, C. C., Kopell, M., McClenahan, K. L., Agras, W. S. & Pfefferbaum, A. (1986). Autonomic characteristics of agoraphobia with panic attacks. Biological Psychiatry, 21, 1133-54.
- Roth, W. T., Wilhem, F. H. & Trabert, W. (1998). Autonomic instability during relaxation in panic disorder. Psychiatry Research, 80, 155-64.
- Siegel, S., Castellan, N. J. Jr. (1988). Nonparametric Statistics for the Behavioral Sciences (2nd ed.). New York: McGraw-Hill
- Stegagno, L. & Palomba, D. (1994). Psicofisiologia clinica. In L. Stegagno (eds.) Psicofisiologia (2): correlati fisiologici dei processi cognitivi e del comportamento. Torino: Bollati Boringhieri.
- Thorell, L. H., Kjellman, B. F. & d’Elia, G. (1987). Electrodermal activity in relation to diagnostic subgroups and symptoms of depressive patients. Acta Psychiatrica Scandinavia, 76 (6), 693-701.
- Thorell L.H. (2009). Valid electrodermal hyporeactivity for depressive suicidal propensity offers links to cognitive theory. Acta Psychiatrica Scandinavia, 119, 338–349.
- Thorell L.H., Wolfersdorf M., Straub R., Steyer J., Hodgkinson S., Kaschka W.P. and Jandl M. (2013). Electrodermal hyporeactivity as a trait marker for suicidal propensity in uni- and bipolar depression. Journal of Psychiatric Research, 47, 1925-1931.
- Ward, N. G. & Doerr, H. O. (1986). Skin conductance. A potentially sensitive and specific marker for depression. Nervous Mental Diseases, 174 (9), 553-9.
- Wilhem, F., Trabert, W. & Roth, W. T. (2001). Physiologic instability in Panic Disorder and Generalized Axiety Disorder. Biological Psychiatry, 49, 596-605.Wise V., Mc Farlane A.C., Clark C.R. and Battersby M. (2011). An integrative assessment of brain and body function ‘at rest’ in panic disorder: A combined quantitative EEG/autonomic function study. International Journal of Psychophysiology 79, 155–165
- Zahn, T. P., Leonard, H. L., Swedo, S. E. & Rapaport, J. L. (1996). Autonomic activity in children and adolescents with obsessive-compulsive disorder. Psychiatry Research, 60, 67-76.
Note
Surgeon: Centro Medico Don Bosco s.n.c., Viareggio, Italy.









